
°æƒø°ø£®ªØ—ߣ≠ŒÔ÷ Ω·ππ”Ζ‘÷ £©
£®1£©“—÷™A∫ÕBŒ™µĞ»˝÷İ∆Ğ‘™Àÿ£¨∆‰‘≠◊”µƒµĞ“ª÷¡µĞÀƒµÁ¿Îƒİ»Áœ¬±ÌÀ˘ æ£∫
µÁ¿Îƒİ/kJ°§mol£≠1 | I1 | I2 | I3 | I4 |
A | 578 | 1817 | 2745 | 11578 |
B | 738 | 1451 | 7733 | 10540 |
AÕ®≥£œ‘_____ºğ£¨AµƒµÁ∏∫–‘________BµƒµÁ∏∫–‘£®ÃÓ°∞£æ°±°¢°∞£º°±ªÚ°∞£Ω°±£©°£
£®2£©◊œÕ‚π‚µƒπ‚◊”À˘æş”–µƒƒİ¡ø‘ºŒ™399 kJ°§mol£≠1°£∏˘æıœ¬±Ì”–πÿµ∞∞◊÷ ∑÷◊”÷–÷ÿ“™ªØ—ߺ¸µƒ–≈œ¢£¨Àµ√˜»ÀÃÂ≥§ ±º‰’’…‰◊œÕ‚π‚∫Û∆§∑Ù“◊ İ…À∫¶µƒ‘≠“Ú__________________________________________________________________________°£
π≤ºğº¸ | C£≠C | C£≠N | C£≠S |
º¸ƒİ/ kJ°§mol£≠1 | 347 | 305 | 259 |
◊È≥…µ∞∞◊÷ µƒ◊ÓºÚµ•µƒ∞±ª˘À·÷–µƒÃº‘≠◊”‘”ªØ¿‡–Õ «________________°£
£®3£© µ—È÷§√˜£∫KCl°¢MgO°¢CaO°¢TiN’‚4÷÷æßõƒΩ·ππ”ÎNaClæßÃÂΩ·ππœ‡À∆£®»ÁÕºÀ˘ 棩£¨
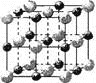
∆‰÷–3÷÷¿Î◊”æßõƒæß∏Òƒİ ˝æı»Áœ¬±Ì£∫
¿Î◊”æßà| NaCl | KCl | CaO |
æß∏Òƒİ/kJ°§mol£≠1 | 786 | 715 | 3401 |
‘Ú∏√ 4÷÷¿Î◊”æßã®≤ª∞¸¿®aCl£©»ğµ„¥”∏şµΩµÕµƒÀ≥–Ú «£∫_________________°£
∆‰÷–MgOæßÃÂ÷–“ª∏ˆMg2+÷İŒß∫ÕÀ¸◊Ó¡ĞΩ¸«“µ»æ‡¿ÎµƒMg2+”–_______∏ˆ°£
£®4£©Ω ٗٿÎ◊”∫¨Œ¥≥…∂‘µÁ◊”‘Ω∂‡£¨‘Ú¥≈–‘‘Ω¥Û£¨¥≈º«¬º–‘ƒİ‘Ω∫√°£¿Î◊”–Õ—ªØŒÔV2O5∫ÕCrO2÷–£¨ ∫œ◊˜¬º“Ù¥¯¥≈∑ğ‘≠¡œµƒ «_____________°£
£®5£©ƒ≥≈‰∫œŒÔµƒ∑÷◊”Ω·ππ»Á”“ÕºÀ˘ 棨∆‰∑÷◊”ƒĞ≤ª∫¨”–____£®ÃÓ–Ú∫≈£©°£
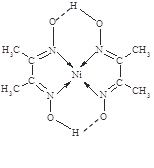
A£Æ¿Î◊”º¸ B£Æº´–‘º¸
C£ÆΩ Ùº¸ D£Æ≈‰Œªº¸
E£Æ«‚º¸ F£Æ∑«º´–‘º¸
£®6£©Œ¬ “–ß”¶£¨ø∆—ߺ“…˺∆∑¥”¶£∫CO2+4H2°˙CH4+2H2O“‘º–°ø’∆¯÷–CO2°£
»Ù”–1mol CH4…˙≥…£¨‘Ú”–______mol¶“º¸∫Õ______mol¶–º¸∂œ¡—°£
°æ¥∞∏°ø+3 £æ ◊œÕ‚π‚æş”–µƒƒİ¡ø±»µ∞∞◊÷ ∑÷◊”÷–µƒªØ—ߺ¸C£≠C°¢C£≠N°¢C£≠Sµƒº¸ƒİ¥Û£¨◊œÕ‚π‚µƒƒİ¡ø◊„“‘ π’‚–©º¸∂œ¡—£¨¥”∂¯∆∆ªµµ∞∞◊÷ ∑÷◊” sp2°¢sp3 TiN£æMgO£æCaO£æKCl 12 CrO2 AC 6 2
°æΩ‚Œˆ°ø
£®1£©A∫ÕBæ˘Œ™µĞ»˝÷İ∆Ğ‘™Àÿ£¨∏˘æı∆‰÷º∂µÁ¿Îƒİø…»∑∂®À¸√«µƒªØ∫œºğ£¨Ω¯∂¯»∑∂®À¸√«µƒµÁ∏∫–‘°£
£®2£©µ∞∞◊÷ ∑÷◊”÷–µƒªØ—ߺ¸µƒƒİ¡ø∫Õ◊œÕ‚π‚µƒπ‚◊”À˘æş”–µƒƒİ¡ø±»Ωœø…÷™◊œÕ‚π‚∂‘»Àà«…À∫¶°£◊È≥…µ∞∞◊÷ µƒ◊ÓºÚµ•µƒ∞±ª˘À· «∏ ∞±À·H2NCH2COOH£¨∏˘æı∑÷◊”÷–µƒªØ—ߺ¸ø…÷™Ãº‘≠◊”‘”ªØ¿‡–Õ°£
£®3£©æß∏Òƒİ∫Õ¿Î◊”∞Îæ∂“‘º∞¿Î◊”À˘¥¯µÁ∫…”–πÿ£¨æß∏Òƒİ‘Ω¥Û£¨»ğ∑–µ„‘Ω∏ş°£∑¬’’NaClµƒæßÃÂΩ·ππø…÷™MgOæßÃÂ÷–“ª∏ˆMg2+÷İŒß∫ÕÀ¸◊Ó¡ĞΩ¸«“µ»æ‡¿ÎµƒMg2+µƒ∏ˆ ˝°£
£®4£©∏˘æı‘≠◊”∫ÀÕ‚µÁ◊”≈≈≤ºø…÷™V∫ÕCr÷–µƒŒ¥≥…∂‘µÁ◊”°£
£®5£©∏˘æı≈‰∫œŒÔµƒ∑÷◊”Ω·ππ£¨ø…÷™∆‰∑÷◊”ƒĞ≤ª∫¨”–µƒ◊˜”√¡¶°£
£®6£©∑¥”¶÷–CO2+4H2°˙CH4+2H2O£¨”–1mol CH4…˙≥…£¨‘Úœ˚∫ƒ1molCO2∫Õ4molH2°£
£®1£©AµƒµĞÀƒµÁ¿ÎƒİÃÿ±¥Û£¨À˘“‘AÕ®≥£œ‘£´3ºğ°£∂¯BµƒµĞ»˝µÁ¿ÎƒİÃÿ±¥Û£¨À˘“‘Bœ‘£´2ºğ£¨À˘“‘Bµƒ‘≠◊”–Ú ˝–°”ĞAµƒ£¨À˘“‘µÁ∏∫–‘Bµƒ–°”ĞAµƒ°£
£®2£©∏˘æıÀ˘∏¯ ˝æıø…÷™£¨◊œÕ‚π‚æş”–µƒƒİ¡ø±»µ∞∞◊÷ ∑÷◊”÷–µƒªØ—ߺ¸C£≠C°¢C£≠N°¢C£≠Sµƒº¸ƒİ¥Û£¨◊œÕ‚π‚µƒƒİ¡ø◊„“‘ π’‚–©º¸∂œ¡—£¨¥”∂¯∆∆ªµµ∞∞◊÷ ∑÷◊”£¨ π»ÀÃÂ İµΩ…À∫¶°£◊ÓºÚµ•µƒ∞±ª˘À· «∏ ∞±À·£¨Ω·ππºÚ ΩŒ™H2NCH2COOH£¨∆‰÷–∫Õ∞±ª˘œ‡¡¨µƒÃº‘≠◊”µƒªØ—ߺ¸»´≤ø «µ•º¸£¨À˘“‘ «sp3‘”ªØ£¨Ù»ª˘÷–µƒÃº‘≠◊”∫¨”–À´º¸£¨ Ù”Ğsp2‘”ªØ°£
£®3£©”∞œÏ¿Î◊”æßûğµ„∏şµÕµƒ «¿Î◊”º¸µƒ«ø»£¨∂¯”∞œÏ¿Î◊”º¸«ø»µƒ «–Œ≥…¿Î◊”º¸µƒ“—Ù¿Î◊”µƒ∞Îæ∂¥Û–°∫ÕµÁ∫… ˝µƒ∂‡…Ÿ°£‘ĞTiN÷–“—Ù¿Î◊”µƒµÁ∫… ˝◊Ó∂‡£¨“¿Î◊”µƒ∞Îæ∂◊Ó–°£¨À˘“‘¿Î◊”º¸◊Ó«ø£¨»ğµ„◊Ó∏ş°£√æ¿Î◊”∞Îæ∂–°”Ğ∏∆¿Î◊”µƒ£¨À˘“‘—ªØ√浃»ğµ„∏ş”Ğ—ªØ∏∆µƒ£¨∏˘æıæß∏҃ݥۖ°ø…÷™£¨—ªØ∏∆µƒ»ğµ„”¶∏√∏ş”Ь»ªØºÿµƒ°£∏˘æı¬»ªØƒ∆µƒæß∞˚ø…÷™“ª∏ˆNa+÷İŒß∫ÕÀ¸◊Ó¡ĞΩ¸«“µ»æ‡¿ÎµƒNa+”–12∏ˆ£¨À˘“‘MgOæßÃÂ÷–“ª∏ˆMg2+÷İŒß∫ÕÀ¸◊Ó¡ĞΩ¸«“µ»æ‡¿ÎµƒMg2+“≤”–12∏ˆ°£
£®4£©∏˘æıππ‘Ï‘≠¿Ìø…÷™£¨Ω ÙV∫ÕCr÷–∫¨”–µƒŒ¥≥…∂‘µÁ◊”∑÷±Œ™3∫Õ6£¨À˘“‘ ∫œ◊˜¬º“Ù¥¯¥≈∑ğ‘≠¡œµƒ «CrO2°£
£®5£©∏˘æıΩ·ππºÚ Ωø…÷™Ãº‘≠◊”∫Õú‘≠◊”–Œ≥…∑«º´–‘º¸£¨Ãº∫Õ«‚–Œ≥…º´–‘º¸£¨µ™‘≠◊”∫ÕNi–Œ≥…≈‰Œªº¸£¨¡ÌÕ‚—∫Õ«‚ªπ”–«‚º¸£¨≤ª¥Ê‘Ğ¿Î◊”º¸∫ÕΩ Ùº¸°£
£®6£©«‚∆¯÷–»´≤ø «¶“º¸°£CO2÷–∫¨”–2∏ˆÀ´º¸£¨À´º¸ «”…1∏ˆ¶“º¸∫Õ1∏ˆ¶–º¸ππ≥…µƒ°£À˘“‘”–1mol CH4…˙≥…£¨‘Ú”–6ml¶“º¸∂œ¡—£¨2mol¶–º¸∂œ¡—°£



| ƒÍº∂ | ∏ş÷–øŒ≥à | ƒÍº∂ | ≥÷–øŒ≥à |
| ∏ş“ª | ∏ş“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥“ª | ≥“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏ş∂˛ | ∏ş∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥∂˛ | ≥∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏ş»˝ | ∏ş»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥»˝ | ≥»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡–”–πÿµÁΩ‚÷ »İ“∫µƒ– ˆ’˝»∑µƒ «£® £©
A.Œ™»∑∂®![]() ««øÀ·ªπ «»À·£¨ø…≤‚NaHA»İ“∫µƒpH£¨»Ù
««øÀ·ªπ «»À·£¨ø…≤‚NaHA»İ“∫µƒpH£¨»Ù![]() £¨‘Ú
£¨‘Ú![]() «»À·£ª»Ù
«»À·£ª»Ù![]() £¨‘Ú
£¨‘Ú![]() ««øÀ·
««øÀ·
B.![]() ±£¨‘Ğ∞±ÀÆÃÂœµ÷–≤ª∂œÕ®»Î
±£¨‘Ğ∞±ÀÆÃÂœµ÷–≤ª∂œÕ®»Î![]() £¨ÀÊ◊≈
£¨ÀÊ◊≈![]() µƒÕ®»Î£¨
µƒÕ®»Î£¨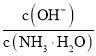 ≤ª∂œº…Ÿ
≤ª∂œº…Ÿ
C.≥£Œ¬œ¬°£Ω´µ»Ãª˝°¢µ»ŒÔ÷ µƒ¡ø≈®∂»µƒ![]() ”ÎNaC1»İ“∫ªÏ∫œ£¨Œˆ≥ˆ≤ø∑÷
”ÎNaC1»İ“∫ªÏ∫œ£¨Œˆ≥ˆ≤ø∑÷![]() æßã¨π˝¬À£¨À˘µ√¬À“∫
æßã¨π˝¬À£¨À˘µ√¬À“∫![]() £¨‘Ú¬À“∫÷–£∫
£¨‘Ú¬À“∫÷–£∫![]()
![]()
D. “Œ¬œ¬£¨Ω´![]()
![]() πÃûݔĞÀÆ≈‰≥…100mL»İ“∫£¨œÚ»İ“∫÷–º”»Î
πÃûݔĞÀÆ≈‰≥…100mL»İ“∫£¨œÚ»İ“∫÷–º”»Î![]() £¨À˘µ√»İ“∫÷–
£¨À˘µ√»İ“∫÷–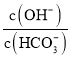 º–°
º–°
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø Ø”Õ∫Õ√∫∂º «÷ÿ“™µƒƒİ‘¥∫ÕªØπ§‘≠¡œ£¨»ÁÕº1 «√∫ªØπ§≤˙“µ¡¥µƒ“ª≤ø∑÷°£

‘”√À˘—ß÷™ ∂£¨Ω‚戜¬¡–Œ £∫
£®1£©≤Ò”Õ «”… ؔպ”π§µ√µΩµƒ÷ÿ“™≤˙∆∑£¨À¸‘Ğ»º…’ ±Õ˘Õ˘√∞∫Зã¨ø…ƒİµƒ‘≠“Ú «____°£
£®2£©√∫æ≠π˝∏…¡Ûø…“‘µ√µΩΩπ¬Ø∆¯°¢√∫Ωπ”Õ∫ÕΩπÃøµ»°£√∫Ωπ”Õæ≠π˝____£®ÃÓº”π§∑Ω∑®£©ø…µ√µΩ∑ºœ„◊ªØ∫œŒÔ°£
£®3£©√∫µƒ÷±Ω”“∫ªØ «√∫∫Õ µ±»İº¡ªÏ∫œ‘Ğ∏şŒ¬∫Õ____¥Ê‘Ğœ¬”Î____◊˜”√…˙≥…“∫軺¡œµƒπ˝≥ð£
£®4£©√∫∫Õ Ø”Õµ»ªØ Ø»º¡œ»º…’≈≈∑≈µƒ¥Û¡ø∂˛—ªØúª·“˝∆»´«Ú∆¯∫Ú±‰≈Ø°£“ª÷÷–¬µƒ¥¶¿Ì∑Ω∑® «Ω´∂˛—ªØú∆¯ÃÂÕ®»Î∫¨”–≥§ Ø£®µÿø«÷–◊Ó≥£º˚µƒøÛ Ø£¨∫¨¡ø∏ş¥Ô60%£©≥…∑÷µƒÀƻݓ∫¿Ô£¨∆‰÷–“ª÷÷∑¥”¶µƒªØ—ß∑Ω≥Ã Ω£∫KAlSi3O8+CO2+2H2O=KHCO3+X°˝+3SiO2°˝£¨‘ÚXµƒªØ—ß ΩŒ™____°£
£®5£©π§“µ…œ÷˜“™≤…”√∞±—ªØ∑®…˙≤˙œÀ·£¨»ÁÕº2 «∞±—ªØ¬ ”Î∞±--ø’∆¯ªÏ∫œ∆¯ÃÂ÷–—∞±±»µƒπÿœµ°£∆‰÷–÷±œş±Ì æ∑¥”¶µƒ¿Ì¬ğ÷µ£ª«˙œş±Ì æ µº …˙≤˙«Èøˆ°£µ±∞±—ªØ¬ ¥ÔµΩ100%£¨¿Ì¬ğ…œ¶√[n£®O2£©/n£®NH3£©]=___£¨ µº …˙≤˙Ω´¶√Œ¨≥÷‘Ğ1.7°´2.2÷ƺ‰£¨‘≠“Ú «____°£

≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø”–πÿ¥şªØº¡µƒ¥şªØª˙¿Ìµ»Œ Âø…“‘¥”°∞““¥º¥şªØ—ªØ µ—È°±µ√µΩ“ª–©»œ ∂,ƒ≥Ωà ¶…˺∆¡À»ÁÕºÀ˘ æ◊∞÷√(º–≥÷◊∞÷√µ»“— °¬‘),∆‰ µ—È≤Ÿ◊˜Œ™:œ»∞¥Õº∞≤◊∞∫√◊∞÷√,πÿ±’ªÓ»˚a°¢b°¢c,‘ĞÕ≠Àøµƒ÷–º‰≤ø∑÷º”»»∆¨øÃ,»ª∫Û¥Úø™ªÓ»˚a°¢b°¢c,Õ®π˝øÿ÷∆ªÓ»˚a∫Õb,∂¯”–ΩĞ◊‡(º‰–™–‘)µÿÕ®»Î∆¯ÃÂ,º¥ø…‘ĞM¥¶πğ≤ϵΩ√˜œ‘µƒ µ—Èœ÷œÛ°£ ‘ªÿ¥“‘œ¬Œ Â:
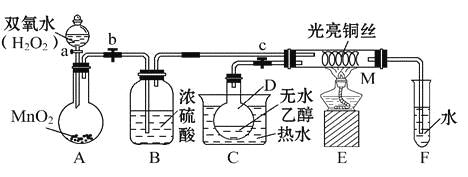
£®1£©A÷–∑¢…˙∑¥”¶µƒªØ—ß∑Ω≥à Ω:__________________,Bµƒ◊˜”√:_____________;C÷–»»ÀƵƒ◊˜”√:_____________________°£
£®2£©M¥¶∑¢…˙∑¥”¶µƒªØ—ß∑Ω≥Ã ΩŒ™_________________°£
£®3£©¥”Mπİ÷–ø…πğ≤ÏµΩµƒœ÷œÛ:_______________,¥”÷–ø…»œ ∂µΩ∏√ µ—Èπ˝≥Ã÷–¥şªØº¡______(ÃÓ°∞≤Œº”°±ªÚ°∞≤ª≤Œº”°±)ªØ—ß∑¥”¶,ªπø…“‘»œ ∂µΩ¥şªØº¡∆¥şªØ◊˜”√–Ë“™“ª∂®µƒ_____________°£
£®4£© µ—ÈΩ¯––“ª∂Œ ±º‰∫Û,»Áπ˚≥∑µÙæ∆æ´µ∆,∑¥”¶__________(ÃÓ°∞ƒİ°±ªÚ°∞≤ªƒİ°±)ºÃ–¯Ω¯––,∆‰‘≠“Ú «_____________°£
£®5£©—È÷§““¥º—ªØ≤˙ŒÔµƒªØ—ß∑Ω∑® «______________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øΩ·π𵃗–æø «”–ª˙ªØ—ß◊Ó÷ÿ“™µƒ—–æø¡Ï”ڣƃ≥”–ª˙ŒÔX£®C12H13O6Br£©∑÷◊”÷–∫¨”–∂‡÷÷πŸƒİÕ≈£¨∆‰Ω·ππºÚ Ω»Áœ¬£∫£®∆‰÷–¢Ò°¢¢ÚŒ™Œ¥÷™≤ø∑÷µƒΩ·π𣩣Æ

Œ™Õ∆≤‚Xµƒ∑÷◊”Ω·ππ£¨Ω¯––»ÁÕº◊™ªØ£∫

“—÷™œÚDµƒÀƻݓ∫÷–µŒ»ÎFeCl3»İ“∫œ‘◊œ…´£¨∂‘DµƒΩ·ππΩ¯––π‚∆◊∑÷Œˆ£¨‘Ğ«‚∫À¥≈π≤’Ò∆◊…œœ‘ æ÷ª”–¡Ω÷÷–≈∫≈£ÆM°¢Nª•Œ™Õ¨∑÷“Ïππã¨M÷–∫¨”–“ª∏ˆ¡˘‘≠◊”ª∑£¨Nƒİ π‰ÂµƒÀƒ¬»ªØú»İ“∫Õ …´£¨Gƒİ”ÎNaHCO3»İ“∫∑¥”¶£Æ«Îªÿ¥£∫
£®1£©G∑÷◊”À˘∫¨πŸƒİÕ≈µƒ√˚≥∆ «__£ª
£®2£©D≤ªø…“‘∑¢…˙µƒ∑¥”¶”–£®—°ÃÓ–Ú∫≈£©__£ª
¢Ÿº”≥…∑¥”¶ ¢Ğœ˚»•∑¥”¶ ¢ğ—ªØ∑¥”¶ ¢İ»°¥˙∑¥”¶
£®3£©–¥≥ˆ…œÕº◊™ªØ÷–∑¥”¶¢Ÿ∫բеƒªØ—ß∑Ω≥à Ω
¢ŸB+F°˙M__£ª
¢ĞG°˙N__£ª
£®4£©“—÷™œÚX÷–º”»ÎFeCl3»İ“∫£¨ƒİ∑¢…˙œ‘…´∑¥”¶£¨‘ÚXµƒΩ·ππºÚ Ω «£∫___________£¨1mol∏√ X”Î◊„¡øµƒNaOH»İ“∫◊˜”√£¨◊Ó∂‡ø…œ˚∫ƒNaOH__mol£ª
£®5£©”–“ª÷÷ªØπ§≤˙∆∑µƒ÷–º‰ÃÂW”ÎGª•Œ™Õ¨∑÷“Ïππã¨Wµƒ∑÷◊”÷–÷ª∫¨”–Ù»ª˘°¢Ù«ª˘∫Õ»©ª˘»˝÷÷πŸƒİÕ≈£¨«“Õ¨“ª∏ˆÃº‘≠◊”…œ≤ªƒİÕ¨ ±¡¨”–¡Ω∏ˆÙ«ª˘£Æ‘ÚWµƒ∑÷◊”Ω·ππ”–__÷÷£¨–¥≥ˆ»Œ“‚“ª÷÷µƒΩ·ππºÚ Ω___________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø‘Ğ25°Ê ±,Ω´1.0L c mol°§L-1 CH3COOH»İ“∫”Î0.1mol NaOHπÃêÏ∫œ£¨ π÷Æ≥‰∑÷∑¥”¶°£»ª∫ÛœÚ∏√ªÏ∫œ»İ“∫÷–Õ®»ÎHCl∆¯Ãªں”»ÎNaOHπÃÃÂ(∫ˆ¬‘ê˝∫ÕŒ¬∂»±‰ªØ)£¨»İ“∫pHÀÊÕ®»Î(ªÚº”»Î)ŒÔ÷ µƒŒÔ÷ µƒ¡øµƒ±‰ªØ»ÁÕºÀ˘ æ°£œ¬¡–– ˆ¥ÌŒÛµƒ «( )
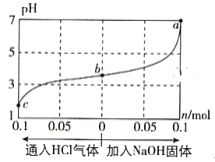
A. ÀƵƒµÁ¿Î≥Ã∂»£∫a>b>c
B. cµ„∂‘”¶µƒªÏ∫œ»İ“∫÷–£∫c(CH3COOH)>c(Na+)>c(OH-)
C. aµ„∂‘”¶µƒªÏ∫œ»İ“∫÷–£∫c(Na+)=c(CH3COO-)
D. ∏√Œ¬∂»œ¬£¨CH3COOHµƒµÁ¿Î∆Ω∫‚≥£ ˝![]()
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø11.2Lº◊ÕÈ°¢““ÕÈ°¢º◊»©◊È≥…µƒªÏ∫œ∆¯Ã£¨ÕÍ»´»º…’∫Û…˙≥…15.68L CO2(∆¯ÃÂê˝æ˘‘бÍ◊º◊¥øˆœ¬≤‚∂®)£¨ªÏ∫œ∆¯ÃÂ÷–““ÕȵƒÃª˝∞Ÿ∑÷∫¨¡øŒ™£® £©
A.80£•B.60£•C.40£•D.20£•
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø≥£Œ¬œ¬£¨œ‡Õ¨≈®∂»µƒ¡Ω÷÷“ª‘™À·HX°¢HY∑÷±”√Õ¨“ª≈®∂»µƒNaOH±Í◊º»İ“∫µŒ∂®£¨µŒ∂®«˙œş»ÁÕºÀ˘ æ°£œ¬¡–Àµ∑®’˝»∑µƒ «![]()
![]()
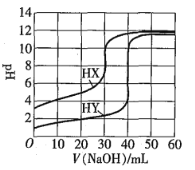
A.HX°¢HY∆ º»İ“∫ê˝œ‡Õ¨
B.æ˘ø…”√º◊ª˘≥»◊˜µŒ∂®÷∏ 溡
C.pHœ‡Õ¨µƒ¡Ω÷÷À·»İ“∫÷–£∫![]()
D.Õ¨≈®∂»KX”ÎHXµƒªÏ∫œ»İ“∫÷–£¨¡£◊”≈®∂»º‰¥Ê‘Ğπÿœµ Ω£∫![]()
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏ş÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡–ÕºœÒ∑÷±±Ì æ”–πÿ∑¥”¶µƒ∑¥”¶π˝≥Ô΃ݡø±‰ªØµƒπÿœµ£¨∆‰÷–≈–∂œ’˝»∑µƒ «( )
A. 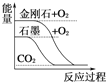 ؃´◊™±‰≥…Ω∏’ Ø «Œ¸»»∑¥”¶
؃´◊™±‰≥…Ω∏’ Ø «Œ¸»»∑¥”¶
B. 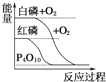 ∞◊¡◊±»∫Ï¡◊Œ»∂®
∞◊¡◊±»∫Ï¡◊Œ»∂®
C. 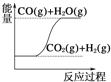 CO(g)+H2O(g)£ΩCO2(g)+H2(g) ¶§H£æ0
CO(g)+H2O(g)£ΩCO2(g)+H2(g) ¶§H£æ0
D. 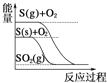 S(g)+O2(g)£ΩSO2(g) ¶§H1 S(s)+O2(g)£ΩSO2(g) ¶§H2,‘Ú¶§H1£æ¶§H2
S(g)+O2(g)£ΩSO2(g) ¶§H1 S(s)+O2(g)£ΩSO2(g) ¶§H2,‘Ú¶§H1£æ¶§H2
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒŞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com