
ЁОЬтФПЁПгаЛњЮяDОпгаЫсадКЭЧПбѕЛЏадЃЌЪЧвЛжжИпаЇЙуЦзЩБОњМСЃЌЦфжЦБИТЗЯпШчЯТЃК
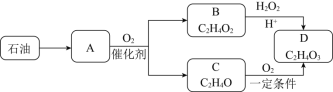
вбжЊЃКAдкБъПіЯТЕФУмЖШЮЊ1.25gL-1ЃЌBОпгаЫсадЃЌCФмЗЂЩњвјОЕЗДгІЁЃЧыЛиД№ЃК
ЃЈ1ЃЉAЕФНсЙЙМђЪН______ЁЃ
ЃЈ2ЃЉCЁњDЕФЗДгІРраЭ______ЁЃ
ЃЈ3ЃЉBЁњDЕФЛЏбЇЗНГЬЪН______ЁЃ
ЃЈ4ЃЉЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ______ЁЃ
AЃЎгЩЪЏгЭЕУЕНAЕФЗНЗЈГЦЮЊСбЛЏ
BЃЎШєAдквЛЖЈЬѕМўЯТФмгыBЗЂЩњМгГЩЗДгІЃЌдђЦфВњЮяПЩФмЮЊввЫсввѕЅ
CЃЎгаЛњЮяDФмЩБОњЯћЖОЕФжївЊдвђЪЧКЌга-COOHНсЙЙ
DЃЎCФмЪЙИпУЬЫсМиЫсадШмвККЭфхЫЎЭЪЩЋЃЌЦфдРэЯрЭЌ
ЁОД№АИЁПCH2=CH2 бѕЛЏЗДгІ CH3COOH+H2O2ЁњCH3COOOH+H2O AC
ЁОНтЮіЁП
AдкБъзМзДПіЯТЕФУмЖШЮЊ1.25gL-1ЃЌдђAЕФФІЖћжЪСПЮЊ1.25g/LЁС22.4L/mol=28g/molЃЌAРДдДгкЪЏгЭЃЌдђAЮЊCH2=CH2ЃЌгЩСїГЬЭМПЩжЊЃЌAбѕЛЏЩњГЩBЃЌBОпгаЫсадЃЌдђBЮЊCH3COOHЃЌAбѕЛЏЩњГЩCЮЊCH3CHOЃЌBгыЙ§бѕЛЏЧтЗЂЩњбѕЛЏЗДгІЩњГЩDЮЊCH3COOOHЃЌCгыбѕЦјЗЂЩњбѕЛЏЗДгІЩњГЩDЁЃ
ЃЈ1ЃЉгЩЩЯЪіЗжЮіПЩжЊЃЌAЕФНсЙЙМђЪНЮЊCH2=CH2ЃЌЙЪД№АИЮЊЃКCH2=CH2ЃЛ
ЃЈ2ЃЉгЩCЁЂDЕФЗжзгЪНПЩжЊЃЌOдзгЪ§діЖрЃЌCЁњD ЕФЗДгІРраЭЮЊбѕЛЏЗДгІЃЌЙЪД№АИЮЊЃКбѕЛЏЗДгІЃЛ
ЃЈ3ЃЉBЁњDЕФЛЏбЇЗНГЬЪНЮЊCH3COOH+H2O2ЁњCH3COOOH+H2OЃЌЙЪД№АИЮЊЃКCH3COOH+H2O2ЁњCH3COOOH+H2OЃЛ
ЃЈ4ЃЉAЃЎгЩЪЏгЭЕУЕНA ЕФЗНЗЈЃЌЮЊБЅКЭДѓЗжзгзЊЛЏЮЊВЛБЅКЭаЁЗжзгЃЌГЦЮЊСбНтЃЌЙЪAДэЮѓЃЛ
BЃЎШє A дквЛЖЈЬѕМўЯТФмгыBЗЂЩњМгГЩЗДгІЃЌдђЦфВњЮяЮЊCH3CH2OOCCH3ЃЌЮЊввЫсввѕЅЃЌЙЪBе§ШЗЃЛ
CЃЎгаЛњЮя D ФмЩБОњЯћЖОЕФжївЊдвђЪЧКЌга-O-O-НсЙЙЃЌОпгаЧПбѕЛЏадЃЌЙЪCДэЮѓЃЛ
DЃЎCЮЊввШЉЃЌКЌ-CHOЃЌФмЪЙИпУЬЫсМиЫсадШмвККЭфхЫЎЭЪЩЋЃЌЦфдРэЯрЭЌЃЌОљЮЊбѕЛЏЗДгІЃЌЙЪDе§ШЗЃЌЙЪД№АИЮЊЃКACЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊЬНЫїЙЄвЕЗЯСЯЕФдйРћгУЃЌФГЛЏбЇаЫШЄаЁзщЩшМЦСЫШчЭМЪЕбщЗНАИЃЌгУКЌгаТСЁЂЬњКЭЭЕФКЯН№жЦШЁТШЛЏТСЁЂТЬЗЏОЇЬхЃЈFeSO47H2OЃЉКЭЕЈЗЏОЇЬхЁЃ
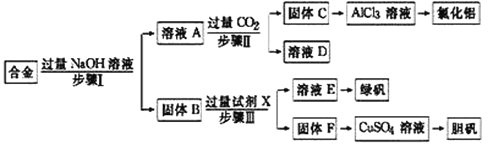
ЧыЛиД№ЃК
ЃЈ1ЃЉВНжшЂёЁЂЂђЁЂЂѓжаОљашНјааЕФЪЕбщВйзїЪЧ_______________ЁЃЪЕбщЪвжаНјааИУВйзїЪБЃЌгУЕНЕФВЃСЇвЧЦїгаЩеБЁЂ______________________ЁЃ
ЃЈ2ЃЉШмвКAжаЕФвѕРызгжївЊга__________________ЃЛгЩКЯН№ЩњГЩAШмвКЕФРызгЗНГЬЪНЮЊЃК_______________ЁЃЪдМСXЪЧ________________ЁЃ
ЃЈ3ЃЉЯђШмвКAжаЭЈШыЙ§СПCO2ЦјЬхЩњГЩЙЬЬхCЕФРызгЗНГЬЪНЮЊ_________________ЁЃ
ЃЈ4ЃЉДгЛЗОГБЃЛЄНЧЖШПМТЧЃЌВЩгУЙЬЬхFМгШызуСПЯЁСђЫсРяМгШШВЂЙФШыПеЦјРДжЦБИCuSO4ШмвКЃЌЦфЛЏбЇЗНГЬЪНЪЧ_______________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЛЏбЇаЫШЄаЁзщгУКЌAЁЂBСНжжН№ЪєЕЅжЪЕФЗлФЉзДЛьКЯЮяНјааШчЯТЪЕбщЃЌЦфзЊЛЏЙиЯЕШчЯТЭМЫљЪОЃЈВПЗжЗДгІЮяКЭЩњГЩЮяЮДСаГіЃЉЃЌЦфжаEЮЊАзЩЋНКзДГСЕэЃЌIЮЊКьКжЩЋГСЕэЁЃЃЈДЫзЊЛЏЙиЯЕжаЫљгУЕФЪдМСЖМЪЧзуСПЕФЃЉ

ЃЈ1ЃЉаДГіЯТСаЮяжЪЕФЛЏбЇЪНЃКF____________ЃЌG________________ЁЃ
ЃЈ2ЃЉНЋЛьКЯЮяжаСНжжН№ЪєЗжРыПЊЕФзюМђЕЅЕФЗНЗЈЪЧ___________ЁЃ
ЃЈ3ЃЉDЁњEЕФзЊЛЏжаЃЌМгШыЙ§СПЕФXПЩФмЪЧ_____________________ЁЃ
A.БЅКЭNaClШмвК B.NaOHШмвК C.АБЫЎ D.Ba(OH)2ШмвК
ЃЈ4ЃЉаДГіЯТСазЊЛЏЕФЛЏбЇЗНГЬЪНЃК
AЁњCЃК______________________________________________ЃЛ
HЁњIЃК_______________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙигкРызгЗНГЬЪНЕФЦРМле§ШЗЕФЪЧ
бЁЯю | РызгЗНГЬЪН | ЦРМл |
A | НЋ1molCl2ЭЈШыЕНКЌ2molFeI2ЕФШмвКжаЃК2Fe2ЃЋЃЋCl2==2Fe3ЃЋ+2ClЃ | е§ШЗЃЛFe2ЃЋЕФЛЙдадЧПгкIЃ |
B | гУЖшадЕчМЋЕчНтMgCl2ШмвК:2Cl-+2H2O | ДэЮѓЃЛгІЩњГЩMg(OH)2ГСЕэ |
C | Й§СПSO2ЭЈШыЕНNaClOШмвКжаЃКSO2ЃЋH2O+ClOЃ===HClO+HSO3Ѓ | е§ШЗЃЛH2SO3ЕФЫсадЧПгкHClO |
D | Mg(HCO3)2ШмвКгызуСПЕФNaOHШмвКЗДгІЃКMg2ЃЋ+2HCO3Ѓ+2OHЃ===MgCO3Ё§+2H2O | е§ШЗЃЛMgCO3БШMg(OH)2ИќФбШм |
A.AB.BC.CD.D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИљОнЗНГЬЪН3Cu+8HNO3(ЯЁ)=3Cu(NO3)2+2NOЁќ+4H2OЃЌЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉгУЫЋЯпЧХЗЈБэЪОЕчзгЕФзЊвЦЕФЗНЯђМАЪ§ФП___ЁЃ
ЃЈ2ЃЉИУЗДгІжаЕФбѕЛЏМСЪЧ___ЃЌЛЙдМСЪЧ__ЁЃ
ЃЈ3ЃЉИУЗДгІжаЕФбѕЛЏВњЮяЪЧ__ЃЌЛЙдВњЮяЪЧ__ЁЃ
ЃЈ4ЃЉИУЗДгІжаЬхЯжСЫЯЁЯѕЫсЕФаджЪга__ЁЂ__ЁЃ
ЃЈ5ЃЉНЋЦфИФЮЊРызгЗДгІЗНГЬЪН__ЁЃ
ЃЈ6ЃЉИУЗДгІжабѕЛЏМСгыЛЙдМСЕФЮяжЪЕФСПжЎБШЮЊ__ЁЃ
ЃЈ7ЃЉШєЗДгІжазЊвЦЕФЕчзгЕФЮяжЪЕФСПЪЧ0.9molЃЌдђЩњГЩNOЕФЬхЛ§ЮЊ__ЃЈБъзМзДПіЃЉЃЌБЛЛЙдЕФЯѕЫсЮЊ__molЁЃ
ЃЈ8ЃЉH2S+H2SO4(ХЈ)=SЁ§+SO2Ёќ+2H2OЃЌдкИУЗДгІжаУПвЛФІЖћH2SO4ВЮМгЗДгІЃЌзЊвЦЕФЕчзгЕФЮяжЪЕФСПЮЊ____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП[ЛЏбЇЁЊЁЊбЁао5ЃКгаЛњЛЏбЇЛљДЁ]ЖЫШВЬўдкДпЛЏМСДцдкЯТПЩЗЂЩњХМСЊЗДгІЃЌГЦЮЊGlaserЗДгІЁЃ
2RЁЊCЁдCЁЊH![]() RЁЊCЁдCЁЊCЁдCЁЊR+H2
RЁЊCЁдCЁЊCЁдCЁЊR+H2
ИУЗДгІдкбаОПаТаЭЗЂЙтВФСЯЁЂГЌЗжзгЛЏбЇЕШЗНУцОпгаживЊМлжЕЁЃЯТУцЪЧРћгУGlaserЗДгІжЦБИЛЏКЯЮяEЕФвЛжжКЯГЩТЗЯпЃК

ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉBЕФНсЙЙМђЪНЮЊ______ЃЌD ЕФЛЏбЇУћГЦЮЊ______ЁЃ
ЃЈ2ЃЉЂйКЭЂлЕФЗДгІРраЭЗжБ№ЮЊ______ЁЂ______ЁЃ
ЃЈ3ЃЉEЕФНсЙЙМђЪНЮЊ______ЁЃгУ1 mol EКЯГЩ1,4ЖўБНЛљЖЁЭщЃЌРэТлЩЯашвЊЯћКФЧтЦј_______molЁЃ
ЃЈ4ЃЉЛЏКЯЮяЃЈ![]() ЃЉвВПЩЗЂЩњGlaserХМСЊЗДгІЩњГЩОлКЯЮяЃЌИУОлКЯЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________________________________ЁЃ
ЃЉвВПЩЗЂЩњGlaserХМСЊЗДгІЩњГЩОлКЯЮяЃЌИУОлКЯЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________________________________ЁЃ
ЃЈ5ЃЉЗМЯуЛЏКЯЮяFЪЧCЕФЭЌЗжвьЙЙЬхЃЌЦфЗжзгжажЛгаСНжжВЛЭЌЛЏбЇЛЗОГЕФЧтЃЌЪ§ФПБШЮЊ3:1ЃЌаДГіЦфжа3жжЕФНсЙЙМђЪН_______________________________ЁЃ
ЃЈ6ЃЉаДГігУ2БНЛљввДМЮЊдСЯЃЈЦфЫћЮоЛњЪдМСШЮбЁЃЉжЦБИЛЏКЯЮяDЕФКЯГЩТЗЯп___________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЖўдЊШѕЫсЃЈМђаДЮЊH2AЃЉШмвКЃЌАДЯТЪНЗЂЩњвЛМЖКЭЖўМЖЕчРыЃКH2A![]() H++HAЃЁЂHAЃ
H++HAЃЁЂHAЃ![]() H++A2ЃЁЃвбжЊЯрЭЌХЈЖШЪБЕФЕчРыЖШІС(H2A)>>ІС(HAЃ)ЃЌЯжгаЯТСаЫФжжШмвКЃК
H++A2ЃЁЃвбжЊЯрЭЌХЈЖШЪБЕФЕчРыЖШІС(H2A)>>ІС(HAЃ)ЃЌЯжгаЯТСаЫФжжШмвКЃК
Ђй0.01molЁЄLЃ1ЕФH2AШмвК
Ђк0.01molЁЄLЃ1ЕФNaHAШмвК
Ђл0.02molЁЄLЃ1ЕФHClгы0.04molЁЄLЃ1ЕФNaHAШмвКЕШЬхЛ§ЛьКЯвК
Ђм0.02molЁЄLЃ1ЕФNaOHгы0.02molЁЄLЃ1ЕФNaHAШмвКЕШЬхЛ§ЛьКЯвК
ЯТСаЫЕЗЈжаВЛе§ШЗЕФЪЧ
A.ШмвКЂмвЛЖЈЯдМюадB.c(H2A)зюДѓЕФЪЧЂл
C.c(A2Ѓ)зюаЁЕФЪЧЂйD.c(H+)зюДѓЕФЪЧЂл
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙлВьЯТСаAЁЂBЁЂCЁЂDЁЂEЮхжжСЃзгЃЈдзгЛђРызгЃЉЕФНсЙЙЪОвтЭМЃЌЛиД№гаЙиЮЪЬтЁЃ
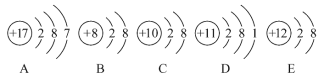
(1)гыРызгЯрЖдгІЕФдЊЫиЕФЗћКХЪЧ_________ЃЌгыдзгЯрЖдгІЕФРызгЕФНсЙЙЪОвтЭМЪЧ__________
(2)ЕчзгВуНсЙЙЯрЭЌЕФЪЧЃЈЬюаДДњКХЃЌдкБОаЁЬтжаЯТЭЌЃЉ_______ЃЌаджЪзюЮШЖЈЕФЪЧ______ЃЌзюШнвзЪЇШЅЕчзгЕФЪЧ_______ЃЌзюШнвзЕУЕНЕчзгЕФЪЧ_______ЁЃ
(3)ПЩжБНгЯрЛЅНсКЯаЮГЩЛЏКЯЮяЕФЛЏбЇЪНЪЧ_______ЃЌПЩОЙ§ЕУЪЇЕчзгКѓдйЯрЛЅНсКЯаЮГЩЛЏКЯЮяЕФЛЏбЇЪНЪЧ__________ЁЃ
(4)дкКЫЕчКЩЪ§1ЁЋ10ЕФдЊЫиФкЃЌСаОйСНИігыBЕчзгВуНсЙЙЯрЭЌЕФРызгЃЌаДГіРызгЕФЗћКХ_______________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЙЄвЕЩЯгУТСЭСПѓЃЈжївЊГЩЗжЮЊAl2O3ЃЌЛЙКЌгаFe2O3ЁЂFeOЁЂSiO2ЃЉжЦБИТСЕФФГжжЛЏКЯЮяЕФЙЄвеСїГЬШчЯТЃЌЯТСагаЙиЫЕЗЈВЛе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
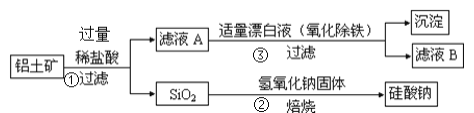
A.ЯђТЫвКAжаМгШыKSCNШмвК,ШмвКвЛЖЈЛсБфКь
B.![]() ЕФРызгЗДгІЗНГЬЪНЮЊ
ЕФРызгЗДгІЗНГЬЪНЮЊ![]()
C.![]() ЦЏАзвКЕФФПЕФЪЧбѕЛЏГ§Ьњ,ИУЙ§ГЬжаЩцМАЕФбѕЛЏЛЙдЗДгІЮЊ
ЦЏАзвКЕФФПЕФЪЧбѕЛЏГ§Ьњ,ИУЙ§ГЬжаЩцМАЕФбѕЛЏЛЙдЗДгІЮЊ![]()
D.![]() жаЦЏАзвКвЊЪЪСП,ШєЙ§СПдђПЩФмВњЩњгаЖОЕФЦјЬхТШЦј
жаЦЏАзвКвЊЪЪСП,ШєЙ§СПдђПЩФмВњЩњгаЖОЕФЦјЬхТШЦј
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com