
����Ŀ����������Ԫ����ɵĻ�����A�����������̽���ʵ�顣
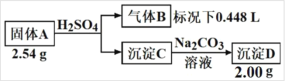
����BΪ����ɫ���嵥�ʣ� ����C��D��ɫ��ӦΪ��Ϊש��ɫ��
(1)���A������Ԫ����______��A�Ļ�ѧʽ��______
(2)����A������ϡ���ᷴӦ�Ļ�ѧ����ʽ��______��
(3)��ӻ�ѧ��Ӧԭ���ĽǶȽ���(��ϻ�ѧ����ʽ)Ϊʲô����C��ת���� D______��
���õIJ����������������ȣ��������������������յ�Ч���������ã�������ˮ�γɵ���ɫ������Һ�������������Ҵ��������Ʊ����������֣�һ����FeCO3�м�������������÷���м��ȡ��������ˮ�������������壬��ʵ����������ͼ��ʾ��
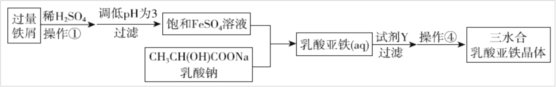
(1)д���Ʊ�����һ�����ӷ�Ӧ����ʽ_______��
(2)�������в���Ҫ�õ�������Ϊ________��
A�������� B������ C������ǯ D��������
(3)����������Լ�YΪ_______��
���𰸡�Ca��O��Cl Ca(ClO)Cl Ca(ClO)Cl+H2SO4=CaSO4+Cl2��+H2O ���������ˮ������Һ�д��ڳ����ܽ�ƽ�⣬����̼���ƣ��ᷢ������ת�����γ�������ˮ��CaCO3����Ӧ����ʽΪ�� 2CH3CH(OH)COOH+FeCO3=2CH3CH(OH)COO-+Fe2++H2O+CO2�� C ��ˮ�Ҵ�
��������
I.(1)����BΪ����ɫ���嵥�ʣ�������ΪCl2��˵��A�к���ClԪ�أ�����������������������ʵ���������������C��D��ɫ��ӦΪ��Ϊש��ɫ��˵������CaԪ�أ�����C��CaSO4������D��CaCO3�����ݳ���D��������A�����������Ԫ�ػ��ϼۣ���ȷ��A�к���H��OԪ�أ���ȷ��A�Ļ�ѧʽ��
(2)�������ʻ�ѧʽ���ܽ��ԣ�д����Ӧ�����ӷ���ʽ��
(3)���ݳ������γɼ�ת��������
II.(1)������������Ա�̼��ǿ�����߷������ֽⷴӦ������ʵ�ǿ�����ܽ��Է�����
(2)���ݷ������ʵĴ���״̬���ܽ��ԡ�Ũ�ȴ�С������������Ϸ����ж�ʹ�õ�������
(3)������������������ˮ�γɵ���ɫ������Һ�������������Ҵ�������������ʡ�
I.(1)n(Cl2)=![]() =0.02 mol��n(Cl)=2n(Cl2)=0.04 mol������m(Cl2)=n��M=0.02 mol��71 g/mol=1.42 g������D��CaCO3����������2.00 g����CaCO3�����ʵ���n(CaCO3)=
=0.02 mol��n(Cl)=2n(Cl2)=0.04 mol������m(Cl2)=n��M=0.02 mol��71 g/mol=1.42 g������D��CaCO3����������2.00 g����CaCO3�����ʵ���n(CaCO3)=![]() =0.02 mol��m(Ca2+)= n��M=0.02 mol��40 g/mol=0.8 g��A������2.54 g�����к��е���һԪ��������m=2.54 g-1.42 g-0.8 g=0.32 g������Ca�������2�����ӣ�Ԫ�ػ��ϼ�Ϊ+2�ۣ�Ca��Cl������һ��Ԫ���γ��Σ������ᷴӦ�������������ʣ�ֻ������H��OԪ�أ�����һ��Ԫ������HԪ�أ������ϻ�������Ԫ�ػ��ϼ۴�����Ϊ0��ԭ��Ԫ�ز�������HԪ�أ�ֻ��ΪOԪ�أ�n(O)=
=0.02 mol��m(Ca2+)= n��M=0.02 mol��40 g/mol=0.8 g��A������2.54 g�����к��е���һԪ��������m=2.54 g-1.42 g-0.8 g=0.32 g������Ca�������2�����ӣ�Ԫ�ػ��ϼ�Ϊ+2�ۣ�Ca��Cl������һ��Ԫ���γ��Σ������ᷴӦ�������������ʣ�ֻ������H��OԪ�أ�����һ��Ԫ������HԪ�أ������ϻ�������Ԫ�ػ��ϼ۴�����Ϊ0��ԭ��Ԫ�ز�������HԪ�أ�ֻ��ΪOԪ�أ�n(O)=![]() =0.02 mol����������A���Ԫ��ΪCa��O��Cl����Ԫ�أ�����A��n(Ca)��n(O)��n(Cl)=0.02��0.02��0.04=1��1��2������A��ѧʽΪCa(ClO)Cl��
=0.02 mol����������A���Ԫ��ΪCa��O��Cl����Ԫ�أ�����A��n(Ca)��n(O)��n(Cl)=0.02��0.02��0.04=1��1��2������A��ѧʽΪCa(ClO)Cl��
(2)����A������ϡ���ᷢ��������ԭ��Ӧ������CaSO4��Cl2��H2O����Ӧ�Ļ�ѧ����ʽ��Ca(ClO)Cl+H2SO4=CaSO4+Cl2��+H2O��
(3)CaSO4����ˮ������Һ�д��ڳ����ܽ�ƽ�⣺CaSO4(s)![]() Ca2+(aq)+SO42-(aq)��������Һ�м���Na2CO3��Һʱ����Һ��c(Ca2+)��c(CO32-)>Ksp(CaCO3)�������˳���ת���γ�CaCO3�������÷�Ӧ�Ļ�ѧ����ʽ��CaSO4+CO32-
Ca2+(aq)+SO42-(aq)��������Һ�м���Na2CO3��Һʱ����Һ��c(Ca2+)��c(CO32-)>Ksp(CaCO3)�������˳���ת���γ�CaCO3�������÷�Ӧ�Ļ�ѧ����ʽ��CaSO4+CO32-![]() CaCO3+SO42-��������ȫת��ΪCaCO3��
CaCO3+SO42-��������ȫת��ΪCaCO3��
II.(1)������������Ա�̼��ǿ����������CH3CH(OH)COOH��FeCO3�������ֽⷴӦ��������������ˮ��������̼�����������ᣬ��Ҫ�Է��Ӵ��ڣ�FeCO3������ˮ����������������ˮ�����Զ��߷������ֽⷴӦ�����ӷ���ʽΪ��2CH3CH(OH)COOH+FeCO3= 2CH3CH(OH)COO-+Fe2++H2O+CO2����
(2)��������������ʹϡ��Һ��ΪŨ�Ƚϴ����Һ���Ӷ�������Һ��pH��ʹ�õ������в�����������ǯ����������Ҫ������Ϊ�������ʺ���ѡ����C��
(3)�����������������������Ҵ�����������ˮ���ʳ��˲���ʱ����ˮ�Ҵ�ϴ�������������塣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и���仯�У�ǰ��С�ں��ߵ���(����)
��CH4(g)��2O2(g)===CO2(g)��2H2O(l)����H1
CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H2
��2H2(g)��O2(g)�T2H2O(l)����H1
H2(g)��![]() O2(g)===H2O(l)����H2
O2(g)===H2O(l)����H2
��t ��ʱ����һ�������£���1 mol SO2��1 mol O2�ֱ����ں��ݺͺ�ѹ�������ܱ������У��ﵽƽ��״̬ʱ�ֱ�Ӧ�ų�������
��CaCO3(s)===CaO(s)��CO2(g)����H1
CaO(s)��H2O(l)===Ca(OH)2(s)����H2
A. �٢ڢ� B. �ڢ� C. �ڢۢ� D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe(NO3)3��һ����Ҫ��ýȾ���ͽ������洦������������ˮ���Ҵ����������ᣬ���н�ǿ�������ԡ�ijѧϰС��������ͼװ���Ʊ�Fe(NO3)3��̽�������ʡ�
�ش��������⣺
����һ���Ʊ�Fe(NO3)3
a�м���100mL8mol��L-1���ᣬb�м���5.6g��м������������м��Ϻ�ˮԡ���ȡ�
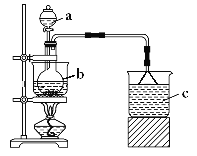
��1������b��������___��
��2����Ӧ�����й۲쵽b����м�ܽ⣬��Һ���ɫ��Һ���Ϸ��к���ɫ�������������b�з�Ӧ�����ӷ���ʽ��___��
��3��c����ʢ�Լ���___������©����������___��
��4����Ҫ�ӷ�Ӧ�����Һ�еõ�Fe(NO3)3���壬Ӧ��ȡ�IJ����ǣ�����Һ����Ũ������ȴ�ᾧ�����ˡ���____ϴ�ӡ����
�������̽��Fe(NO3)3������
i.���0.1mol��L-1Fe(NO3)3��Һ��pHԼ����1.6��
ii.��5mL0.1mol��L-1Fe(NO3)3��Һ�������������Թ��У�Լ1min��������ȫ�ܽ⡣
��5��ʹ�����ܽ��ԭ�������������a.NO3(H+)ʹ�����ܽ⣻b.__ʹ�����ܽ⡣Ϊ֤��b������������·�����ȡ���������ܽ�����Һ����һ֧�Թ��У�___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��֤±�ص��������Ե����ǿ����ijС������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ���)��
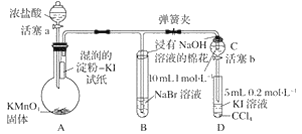
ʵ����̣�
��.���ɼУ�����a���μ�Ũ���ᡣ
��.��B��C�е���Һ����Ϊ��ɫʱ���н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ�غ�ɫʱ���رջ���a��
��.����
��1����֤������������ǿ�ڵ��ʵ��������_________________________________________��
��2��B����Һ������Ӧ�����ӷ���ʽ��____________________________________________��
��3��Ϊ��֤���������ǿ�ڵ⣬�������IJ�����������________________________________��
��4��������ʵ���Ŀ����_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£���Fe2O3��NiO��Cr2O3��������ȼú�������л��գ�ʹSO2ת������S��������ͬ������������ͬ(Ũ�ȡ��¶ȡ�ѹǿ)����£� ��ͬʱ����SO2��ת�����淴Ӧ�¶ȵı仯����ͼ������˵������ȷ����
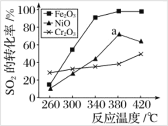
A.�����Ǵ����۸����أ�ѡ��Fe2O3���������Խ�Լ��Դ
B.ѡ��Fe2O3���������������¶�Ϊ340 ������
C.a ���SO2��ת���ʼ�С��ԭ��������¶����ߴ������Խ�����
D.����������ͬ������£�ѡ��Cr2O3��������SO2��ƽ��ת������С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���Ҳ������з�����ȡNH3��
�ٹ���Ca(OH)2��NH4Cl���ȡ�
��NH4HCO3�����м�NaOH����(����)�������¼��ܲ���NH3��װ����ͼ��
![]()
��NaOH�����м���Ũ��ˮ��
��NH4ClŨ��Һ�м���ʯ�ҡ�
��ش��������⣺
(1)д���ڷ�����ȡ�����Ļ�ѧ����ʽ___________________________��
(2)˵���۷�����ȡ������ԭ����_____________��Ϊʲô�˷������ȣ� __________��
(3)��������������ѡ��ܷ�����ȡ�����ķ���װ��________(д��ĸ��Ҫ��ʹ���������١�����)��

д����NH4ClŨ��Һ����ʯ�ҷ�Ӧ��ȡ���������ɣ�__________��

(4)ijͬѧ�������ͼװ���ռ�����������˵������ȷ����________(����ĸ)��
A���ռ�������ԭ���������ſ�����
B������ܵ������Ƿ�ֹ����
C�����ձ��е�ˮ���ʱ֤���������ռ���
D����װ�û��γɺ�ɫ��Ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������(chemical oxygen demand�����COD)��ʾ��ǿ�����������ظ�������� 1 L ��ˮ���л���������������������������������Ϊ������ʱ��1 Lˮ��������O2������(mg��L-1)���㡣CODС��ˮ�ʺá�ij������ֳೱ��ij��ѧ��ȤС��Ϊ�ⶨ����Ⱦ�̶ȣ��� 1.176 g K2Cr2O7�������Ƴ� 100 mL��Һ����ȡˮ��20.00 mL������10.00 mL K2Cr2O7��Һ��������������ʹ��������ȷ�Ӧ2 h�������K2Cr2O7��0.100 0 mol��L-1Fe(NH4)2(SO4)2��Һ���еζ�������Fe(NH4)2(SO4)2��Һ��������±���ʾ����ʱ�������ķ�Ӧ��CrO72-+6Fe2��+14H+=2Cr3��+6Fe3++7H2O��(��֪K2Cr2O7���л��ﷴӦʱ����ԭΪ Cr3+��K2Cr2O7����Է�������Ϊ294)
��� | ��ʼ����/mL | �յ����/mL |
1 | 0.00 | 12.10 |
2 | 1.26 | 13.16 |
3 | 1.54 | 14.64 |
(1)K2Cr2O7��Һ�����ʵ���Ũ��Ϊ______mol��L-1��
(2)��ú�ˮ��CODΪ______mg��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�ĵ��װ�ÿ�ʵ�ֵ͵�λ�¸�Ч����ԭCO2������˵������ȷ����(����)
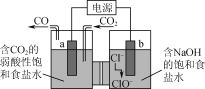
A.a��������ӵ�Դ�ĸ���
B.��������Na�����ҳ��������
C.b���ĵ缫��ӦʽΪCl����2e����H2O=ClO����2H��
D.���·��ÿת��1 mol���ӣ����ۿɴ���ԭ�����CO2����11.2 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ĺ��������������Ϊ��Ǧ���͵Ŀ��������������Է�������������100����C����������Ϊ68.2%����H����������Ϊ13.6%������Ϊ���������ش�
��1���û��������Է���������____________________��
��2��д���û�����ķ���ʽ___________________________��
��3�����û����ﲻ�����Ʒ�Ӧ����������������ͺ˴Ź���������ʾ�÷�������4��������д����ṹ��ʽ��_________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com