
ЎҫМвДҝЎҝAЎўBЎўCЎўDЎўEОеЦЦМю·ЦЧУЦРҫщә¬УР8ёцЗвФӯЧУЈ¬ЖдЦРAЎўBіЈОВПВіКЖшМ¬Ј¬CЎўDЎўEіКТәМ¬ЎЈЗл»Шҙр
(1)AКЗ·ыәПЙПКцМхјюЦРПа¶Ф·ЦЧУЦКБҝЧоРЎөДЈ¬ФтAөД·ЦЧУКҪОӘ________Ј»BКфУЪБҙЧҙөҘП©МюЈ¬ЗТУлHBrјУіЙөДІъОпЦ»УРТ»ЦЦЈ¬КФРҙіцBөДҪб№№јтКҪ________ЎЈ
(2)CКфУЪ·јПгМюЈ¬ҝЙУГАҙЦЖБТРФХЁТ©Ј¬КФРҙіцCЦЖИЎБТРФХЁТ©өД·ҪіМКҪ_______________________________ЎЈ
ЈЁ3Ј©ТСЦӘEөДҪб№№јтКҪОӘ![]() ҙжФЪУЪГәҪ№УНЦРЈ¬ЛщУРФӯЧУҫщҙҰУЪН¬Т»ЖҪГжДЪЈ¬Фт1mol E Чо¶аДЬәН___ molөДH2јУіЙЈ¬РҙіцEөДЛщУРТ»ВИҙъОпөДҪб№№јтКҪ_____________________________ЎЈ
ҙжФЪУЪГәҪ№УНЦРЈ¬ЛщУРФӯЧУҫщҙҰУЪН¬Т»ЖҪГжДЪЈ¬Фт1mol E Чо¶аДЬәН___ molөДH2јУіЙЈ¬РҙіцEөДЛщУРТ»ВИҙъОпөДҪб№№јтКҪ_____________________________ЎЈ
Ўҫҙр°ёЎҝЈЁ1Ј©C3H8CH3CHЈҪCHCH3
ЈЁ2Ј©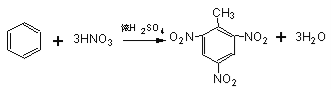
ЈЁ3Ј©5moL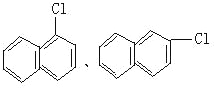
ЎҫҪвОцЎҝ
ЈЁ1Ј©AКЗ·ыәПЙПКцМхјюЦРПа¶Ф·ЦЧУЦКБҝЧоРЎөДЈ¬ТӘК№ЖдПа¶Ф·ЦЧУЦКБҝЧоРЎЈ¬Фтә¬УРөДМјФӯЧУёцКэЧоЙЩЈ¬ЛщТФёГОпЦККЗұыНйЈ¬Жд·ЦЧУКҪОӘЈәC3H8Ј»BКфУЪБҙЧҙөҘП©МюЈ¬ЗТУлHBrјУіЙөДІъОпЦ»УРТ»ЦЦЈ¬ЗТКфУЪЖшМ¬өДә¬УР8ёцЗвФӯЧУөДП©МюОӘ2-¶ЎП©Ј¬ЖдҪб№№јтКҪОӘЈәCH3CH=CHCH3Ј»ЈЁ2Ј©CКфУЪ·јПгМюЈ¬ҝЙУГАҙЦЖБТРФХЁТ©Ј¬ЗТә¬УР8ёцЗвФӯЧУЈ¬ФтCКЗјЧұҪЈ¬јЧұҪәНЕЁПхЛб·ўЙъИЎҙъ·ҙУҰЙъіЙ2Ј¬4Ј¬6-ИэПх»щјЧұҪЈ¬ёГ·ҙУҰ·ҪіМКҪОӘ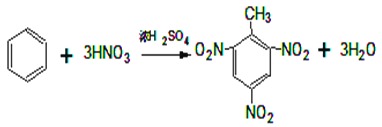 Ј»ЈЁ3Ј©EҙжФЪУЪГәҪ№УНЦРЛөГчә¬УРұҪ»·Ј¬EЦРЛщУРФӯЧУҫщҙҰУЪН¬Т»ЖҪГжДЪЛөГчEЦРІ»ә¬Ц§БҙЈ¬1molEЧо¶аДЬәН5molH2јУіЙЛөГчә¬УР2ёцұҪ»·Ј¬ЗТБҪёцұҪ»·УРБҪёцМјФӯЧУПаБ¬Ј¬EЦРә¬УР8ёцЗвФӯЧУЈ¬Ҫб№№ёЯ¶И¶ФіЖЈ¬Ц»УРБҪЦЦ»ҜС§»·ҫіПВөДЗвЈ¬№КEөДТ»ВИҙъОпУРБҪЦЦЈ¬Ҫб№№јтКҪ·ЦұрОӘЈә
Ј»ЈЁ3Ј©EҙжФЪУЪГәҪ№УНЦРЛөГчә¬УРұҪ»·Ј¬EЦРЛщУРФӯЧУҫщҙҰУЪН¬Т»ЖҪГжДЪЛөГчEЦРІ»ә¬Ц§БҙЈ¬1molEЧо¶аДЬәН5molH2јУіЙЛөГчә¬УР2ёцұҪ»·Ј¬ЗТБҪёцұҪ»·УРБҪёцМјФӯЧУПаБ¬Ј¬EЦРә¬УР8ёцЗвФӯЧУЈ¬Ҫб№№ёЯ¶И¶ФіЖЈ¬Ц»УРБҪЦЦ»ҜС§»·ҫіПВөДЗвЈ¬№КEөДТ»ВИҙъОпУРБҪЦЦЈ¬Ҫб№№јтКҪ·ЦұрОӘЈә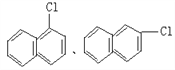 ЎЈ
ЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝИф°ў·ьјУөВВЮіЈКэөДКэЦөУГNAұнКҫЈ¬ПВБРЛө·ЁХэИ·өДКЗЈЁ Ј©
A.1.6gNH2-АлЧУЛщә¬ЦКЧУКэОӘNA
B.іЈОВіЈС№ПВЈ¬200g8.5%өДH2O2ИЬТәЦРә¬СхФӯЧУКэОӘNA
C.7.8gNa2O2ҫ§МеЦРЛщә¬АлЧУЧЬКэОӘ0.4NA
D.ФЪ·ҙУҰKIO3+6HI=KI+3I2+3H2OЦРЈ¬ГҝЙъіЙ3molI2ЧӘТЖөДөзЧУКэОӘ5NA
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝУЙПВБРФӘЛШРОіЙөДөҘЦКЈ¬І»ДЬКөПЦИзПВЧӘ»Ҝ№ШПөөДКЗЈә
өҘЦК![]() Сх»ҜОп
Сх»ҜОп![]() ЛбЈЁ»тјоЈ©
ЛбЈЁ»тјоЈ©![]() СО
СО
A. CB. SC. NaD. Al
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝЈЁ1Ј©4.8gO3әН0.2molCH4Ј¬ФЪН¬ОВН¬С№ПВөДМе»эЦ®ұИКЗ__Ј»ПаН¬ЦКБҝөДO2әНSO2Ј¬Лщә¬·ЦЧУөДКэДҝЦ®ұИОӘ__Ј¬Лщә¬OФӯЧУөДОпЦКөДБҝЦ®ұИОӘ__Ј¬ГЬ¶ИЦ®ұИОӘ__ЎЈ
ЈЁ2Ј©ұкЧјЧҙҝцПВ11.2 L N2Лщә¬·ЦЧУКэКЗ___ёцЎЈ
ЈЁ3Ј©ИфДіФӯЧУөДДҰ¶ыЦКБҝКЗM g/molЈ¬ФтТ»ёцёГФӯЧУөДХжКөЦКБҝКЗ__gЎЈ
ЈЁ4Ј©483g Na2SO4ЎӨ10H2OЦРЛщә¬өДNa+өДОпЦКөДБҝКЗ__Ј¬SO42-өДОпЦКөДБҝКЗ__Ј¬Лщә¬H2O·ЦЧУөДКэДҝКЗ___ёцЎЈ
ЈЁ5Ј©ПЦУР100 ml 1.00 mol/L NaClИЬТәЈ¬ЖдЦРЛщә¬Na+өДЦКБҝКЗ___gЎЈ
ЈЁ6Ј©ПВБРОпЦКДЬөјөзКЗ__Ј¬КфУЪөзҪвЦККЗ__Ј¬КфУЪ·ЗөзҪвЦКөДКЗ__ЎЈЈЁМоРтәЕЈ¬¶аСЎ»тҙнСЎҝЫ·ЦЈ©ўЩЛ®Тш ўЪЙХјо ўЫБтЛбұө ўЬВИ»ҜДЖҫ§Ме ўЭСОЛб ўЮХбМЗ
ЈЁ7Ј©КөСйКТЕдЦЖ480mL0.08mol/LNa2CO3ИЬТәЈ¬РиТӘК®Л®МјЛбДЖҫ§МеЦКБҝ_______gЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРАлЧУ·ҪіМКҪЦРЈ¬ХэИ·өДКЗ (ЎЎЎЎ)
A. ПЎБтЛбөОФЪМъЖ¬ЙПЈә2FeЈ«6HЈ«===2Fe3Ј«Ј«3H2Ўь
B. МјЛбЗвДЖИЬТәУлПЎСОЛб»мәПЈәHCO3-+H+=H2O+CO2Ўь
C. БтЛбНӯИЬТәУлЗвСх»ҜДЖИЬТә»мәПЈәCuSO4Ј«2OHЈӯ===Cu(OH)2ЎэЈ«![]()
D. ПхЛбТшИЬТәУлВИ»ҜДЖИЬТә»мәПЈәAgNO3Ј«ClЈӯ===AgClЎэЈ«![]()
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝёщҫЭПВГжөД·ҙУҰВ·ПЯј°ЛщёшРЕПўМоҝХ

ЈЁ1Ј©AөДҪб№№јтКҪКЗ____________________
ЈЁ2Ј©ўЩөД·ҙУҰАаРНКЗ_________________ЎЈўЫөД·ҙУҰАаРНКЗ__________________ЎЈ
ЈЁ3Ј©·ҙУҰўЬөД»ҜС§·ҪіМКҪКЗ ____________________________________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПЦУРИэЦЦҝЙИЬРФОпЦКAЎўBЎўCЈ¬ЖдЦРAЎўBКфУЪСОЈ¬CКфУЪјоЈ¬ЛьГЗИЬУЪЛ®әуөзАлІъЙъөДЛщУРАлЧУИзПВұнЛщКҫЈә
СфАлЧУ | NaЈ« HЈ« Ba2Ј« |
ТхАлЧУ | OHЈӯ CO32Јӯ SO42Јӯ |
ЗлёщҫЭПВБРРрКц»ШҙрОКМвЈә
ЈЁ1Ј©CөД»ҜС§КҪОӘ___ЎЈ
ЈЁ2Ј©AИЬТәУлBИЬТә·ҙУҰҝЙЙъіЙЖшМеXЈ¬ФтXөД»ҜС§КҪОӘ___Ј¬ёГ·ҙУҰөДАлЧУ·ҪіМКҪОӘ___ЎЈ
ЈЁ3Ј©AЎўBИЬТәУлCИЬТә·ҙУҰҝЙ·ЦұрЙъіЙ°ЧЙ«іБөнDәНEЈ¬ЖдЦРDҝЙИЬУЪПЎПхЛбЎЈ
ўЩBөД»ҜС§КҪОӘ___Ј¬јшұрИЬТәЦРBөДТхАлЧУөДКФјБОӘ___ЎЈ
ўЪDИЬУЪПЎПхЛбөДАлЧУ·ҪіМКҪОӘ___ЎЈ
ўЫDУлEөД»мәПОпagЈ¬јУИлЧгБҝСОЛбЈ¬НкИ«·ҙУҰЙъіЙөДЖшМеФЪұкЧјЧҙҝцПВМе»эОӘVLЈ¬ФтEФЪ»мәПОпЦРөДЦКБҝ·ЦКэөДұнҙпКҪОӘ___ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝОӘБЛҪ«»мУРNa2SO4ЎўNaHCO3өДNaCl№ММеМбҙҝЈ¬ЦЖөГҙҝҫ»өДВИ»ҜДЖИЬТәЈ¬ДіС§ЙъЙијЖБЛИзПВНјЛщКҫөДКөСй·Ҫ°ёЈә

ТСЦӘМјЛбЗвДЖФЪјУИИМхјюПВДЬ·ЦҪвіЙМјЛбДЖЎў¶юСх»ҜМјәНЛ®Ј¬¶шNaClәНNa2SO4№ММејУИИКұІ»·ўЙъ»ҜС§ұд»ҜЎЈЗл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ІЩЧчўЩКў·ЕТ©Ж·ҝЙСЎУГ___________ЈЁМоТЗЖчГыіЖЈ©ЎЈ
ЈЁ2Ј©ІЩЧчўЪОӘКІГҙІ»УГBa(NO3)2ИЬТәЈ¬ЖдАнУЙКЗЈә___________________________ЎЈ
ЈЁ3Ј©ҪшРРІЩЧчўЪәуЈ¬ЕР¶ПSO42-ТСіэҫЎөД·Ҫ·ЁКЗЈә______________________________ЎЈ
ЈЁ4Ј©ІЩЧчўЫөДДҝөДКЗЈә____________________________________________________ЎЈ
ЈЁ5Ј©ҙЛЙијЖ·Ҫ°ёКЗ·сСПГЬ___________ЈЁМоЎ°КЗЎұ»тЎ°·сЎұЈ©АнУЙ_______________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРРрКцұд»ҜАнВЫЙПјЖБҝҪб№ыХэИ·өДКЗЈЁ Ј©
A. ұкЧјЧҙҝцПВЈ¬УГә¬ҝХЖшәН°ұЖшМе»эёч°лөДЙХЖҝҪшРРЕзИӘКөСйЈ¬ЛщөГИЬТәөДОпЦКөДБҝЕЁ¶ИФјОӘ![]()
B. ПаН¬ЦКБҝөДБҪ·ЭВБЈ¬·Цұр·ЕИлЧгБҝөД![]() әН
әН![]() ИЬТәЦРід·Ц·ҙУҰЈ¬ПыәДөД
ИЬТәЦРід·Ц·ҙУҰЈ¬ПыәДөД![]() әН
әН![]() Ц®ұИОӘ1ЎГ1
Ц®ұИОӘ1ЎГ1
C. Ҫ«![]() ЦрөОјУИлөҪ
ЦрөОјУИлөҪ![]() ИЬТәЦРЈ¬ІъЙъ
ИЬТәЦРЈ¬ІъЙъ![]() өДМе»эФјОӘ
өДМе»эФјОӘ![]() ЈЁұкЧјЧҙҝцПВЈ©
ЈЁұкЧјЧҙҝцПВЈ©
D. Пт![]() өД
өД![]() ИЬТәЦРНЁИлЧгБҝөД
ИЬТәЦРНЁИлЧгБҝөД![]() ЖшМеЈ¬ЙъіЙ
ЖшМеЈ¬ЙъіЙ![]() іБөн
іБөн
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com