
����Ŀ��
(1)�ڱ�״���£������ʢ�44.8LH2����24gCH4����1molH2O����3.01��1023��N2����������������______������ţ���ͬ������������������________������������______�������С����______���ܶ���С�����˳��Ϊ________________��
(2)0.5molij����A��������40g��A��Ħ������Ϊ_________��
(3)����֮��Ϊ8��7����������O2��CO���������֮��Ϊ_______________����ԭ����֮��Ϊ____________����ͬ�����µ����֮��Ϊ________��
(4) 4.8g̼��һ������������ȼ�գ���Ӧ������CO��CO2��������Ϊ12.8g������ͬ��ͬѹ�£����ɵ�CO��CO2�������Ϊ__________��
(5)��NaHS��MgSO4��NaHSO3��ɵĻ�����У���Ԫ�ص���������Ϊa��������������Ԫ�ص���������Ϊ_______________��
���𰸡��� �� �� �� �٢ڢܢ� ���� ��<��<��<�ۣ� 80 g��mol��1 1��1 2��1 1��1 3��1 1-1.75a��
��������
��1���������״���£�![]() ��
��![]() ��
��![]() ��
��![]() �����ʵ������ٸ���
�����ʵ������ٸ���![]() ��
��![]() ��
��![]() �������ʽ������ʽ��������������ܶȡ�
�������ʽ������ʽ��������������ܶȡ�
��2������![]() ����Ħ��������
����Ħ��������
��3�������![]() ��
��![]() �����ʵ���֮�ȣ����������֮�ȡ���ԭ����֮�ȣ�ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
�����ʵ���֮�ȣ����������֮�ȡ���ԭ����֮�ȣ�ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
��4������̼�����ʵ������������ɵ�![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ��
��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ���з��������
����������![]() ��
��![]() ��ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
��ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
��5��������������ɣ���֪![]() ��
��![]() �����ʽ������24����������������
�����ʽ������24����������������![]() ����
����![]() �Ĺ�ϵ�����
�Ĺ�ϵ�����![]() ��
��![]() ��
��![]() ��
��![]() Ԫ�ص���������������
Ԫ�ص���������������![]() Ԫ�ص�����������
Ԫ�ص�����������
��1��
��״̬�� | ���ʵ��� | ������ | ������ | ���� | ��� | �ܶ� |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�����������Ǣ٣��������������������������Ǣڣ������С�������������ܶ���С����� < �� < �� < ��
��2��![]()
��3��![]() ��
��![]() �����ʵ���֮��Ϊ��
�����ʵ���֮��Ϊ��![]() ���������֮��Ϊ��
���������֮��Ϊ��![]() ����ԭ����֮��Ϊ��
����ԭ����֮��Ϊ��![]() ��ͬ�����µ����֮�ȵ������ʵ���֮�ȣ�Ϊ
��ͬ�����µ����֮�ȵ������ʵ���֮�ȣ�Ϊ![]()
��4��![]() ̼�����ʵ���Ϊ
̼�����ʵ���Ϊ![]() �������ɵ�
�������ɵ�![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ��
��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ����
����![]() �����
�����![]() ��
��![]() ����ͬ��ͬѹ�£�CO��CO2�����֮�ȵ������ʵ���֮�ȣ���
����ͬ��ͬѹ�£�CO��CO2�����֮�ȵ������ʵ���֮�ȣ���![]() ��
��
��5����![]() ��
��![]() ����һ�����壬���ǵ����ʽ������24����������������S����
����һ�����壬���ǵ����ʽ������24����������������S����![]() �Ĺ�ϵ����Ԫ�ص���������Ϊa�����������Ǿ���
�Ĺ�ϵ����Ԫ�ص���������Ϊa�����������Ǿ���![]() ������
������
![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ��Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ����2Mg+O2![]() 2MgO����3Mg+N2
2MgO����3Mg+N2![]() Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2![]() 2MgO+C����Mg+H2O
2MgO+C����Mg+H2O![]() MgO+H2������Mg3N2+6H2O
MgO+H2������Mg3N2+6H2O![]() 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��
�ɹ�ѡ���װ�ú�ҩƷ��ͼ��ʾ��þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ����������������


�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��E�⣬��Ӧѡ���װ�ã�����ĸ���ţ�����Ŀ�ķֱ���_____��
��2�����Ӳ����ʵ��װ�õ������ԡ�ʵ�鿪ʼʱ��������ˮ�Ŀ��أ���������5���Ĵ���ƿѹ�뷴Ӧװ�ã��������������ܵ�˳���ǣ�����ĸ���ţ�___��
��3��ͨ�������ͬʱ��ȼA��Fװ�õľƾ��ƣ���ʵ�����к�Ӱ�죿____��ԭ����____��
��4�������һ��ʵ�飬��֤�����ǵ���þ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ף����Ա���ʳ��ˮ��NH3��CO2Ϊԭ�����Ƶ�NaHCO3����������������ش��������⣺
ij̽���С����������Ƽ�ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ʒ���ʵ�顣һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ����ͼ��ʾ(ͼ�мг֡��̶��õ�����δ����)
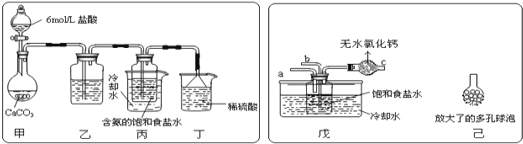
��1����װ����ϡ�����������____��
��2����һλͬѧ��ͼ����װ�ã�����װ��δ����������ʵ�顣ʵ��ʱ�����ȴ�___��ͨ��NH3���塣
��3����ͬѧ��������װ�õ�b���¶����Ӽ�װ�ã�������_____��
��4����������г�������������ڲ�ͬ�¶��µ��ܽ�����ݣ�g/100gˮ����
0�� | 10�� | 20�� | 30�� | 40�� | 50�� | |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
���ձ������ݣ������������װ����ʹ����ȴˮ���߱�ˮ��ԭ��____��
��5����С��ͬѧΪ�˲ⶨ�������þ����̼�����ƵĴ��ȣ����辧���в���̼�������ʣ����������ָ����������Ϊag���ٽ�������ȵ��������ٱ仯ʱ���������÷�ĩ����Ϊmg��Ȼ�������ͼ��ʾʵ�飺
![]()
���ڲ������У�Ϊ���жϼ����Ȼ�����Һ�Ƿ������������ȷ����___������ĸ����
a �ڼ����Ȼ�����Һ�������ã�����Һ�м������������Ȼ�����Һ
b �ڼ����Ȼ�����Һ�������ã�����Һ���ټ�������̼������Һ
c �ڼ����Ȼ�����Һ�������ã�ȡ�ϲ���Һ�ټ�������̼������Һ
�����þ�����̼�����ƵĴ���Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ǿ������ʴ�ԣ�����Ǧ����Ϊ���Դ����Al��������Pb�����������ϡ���ᣬʹ�����������Ĥ�����䷴Ӧԭ�����£� ��أ� Pb(s) + PbO2(s) + 2H2SO4(aq) =2PbSO4(s) + 2H2O(l)��
���أ�2Al+3O2![]() Al2O3+3H2���������У������ж���ȷ���ǣ� ��
Al2O3+3H2���������У������ж���ȷ���ǣ� ��
��� | ���� | |
A | H+����Pb�缫 | H+����Pb�缫 |
B | ÿ����3molPb | ����2molAl2O3 |
C | ������PbO2+4H++2e��=Pb2++2H2O | ������2Al+3H2O-6e��=Al2O3+6H+ |
D |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ֿ���������A��B��C��D��E���������������������ӻ�����ͬ���ֱ�������������Al3����Fe3����Cu2����Ba2����K��������������NO![]() ��OH����Cl����CO32����Xn-(n=1��2)�е�һ�֡�
��OH����Cl����CO32����Xn-(n=1��2)�е�һ�֡�
��1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������____��______��
��2������C�к�������Xn-��Ϊ��ȷ��Xn-���ֽ�(1)�е��������ʼ�ΪA��B����C��A����Һ���ʱ������ɫ��������ó����е�������ϡHNO3�����������ܽ⣬ʣ���ɫ���壨���������ɣ�����CΪ___���������ƣ�
��3����38.4g CuͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�������DΪ_________(��ѧʽ)��д��Cu�ܽ�����ӷ���ʽ_______����Ҫ��Cu��ȫ�ܽ⣬���ټ���H2SO4�����ʵ�����_________��
��4������E��Һ������ᷴӦʱ������ʹ���۱��������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ɡ��ܽ��ת���������Ʊ����ᴿ�Լ����е������й㷺Ӧ�á���֪25��ʱ��Ksp(BaSO4)��1��10��10��Ksp(BaCO3)��1��10��9��
��1����Ũ�Ⱦ�Ϊ0.1 mol��L��1��BaCl2��Һ��Na2SO4��Һ�������ϣ���ֽ������ˣ���Һ��c(Ba2��)��_____mol��L��1��
��2��ҽѧ�Ͻ�������ϵͳ��X������ʱ����ʹ��BaSO4���ڷ���Ӱ����θ�����Ժ�ǿ( pHԼΪ1)�� �����ô���BaSO4��Ȼ�ǰ�ȫ�ģ�BaSO4���������ԭ����_______________________��(�ó����ܽ�ƽ��ԭ������)����һ���������BaCO3��Ӧ�����ô���0.5mol��L��1Na2SO4��Һ������ϴθ���������ϴθ������Na2SO4��ҺŨ�ȵı仯��������θҺ�е�Ba2��Ũ�Ƚ�Ϊ________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����ú������ʣ�FeO��Fe2O3���ķ�CuO�Ʊ��������壬���������й��̣���֪ Fe3+�� pH=5ʱ������ȫ�������з����������

A. ����ڷ�������Ҫ��ӦΪ��2Fe2++H2O2+2H+=2Fe3++2H2O
B. ����ڲ�������ˮ�������ǿ����������H2O2
C. �����Ϊ���ˣ�����������ᾧ
D. ������� CuCO3����CuOҲ�ɵ�����Һ��pH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƾ��壨Na2MoO4��2H2O��������������ȼ������������ˮϵͳ�Ľ������Ƽ�����ͼ�������⾫����Ҫ�ɷ���MoS2��������PbS�ȣ�Ϊԭ�����������ƾ���Ĺ�������ͼ��
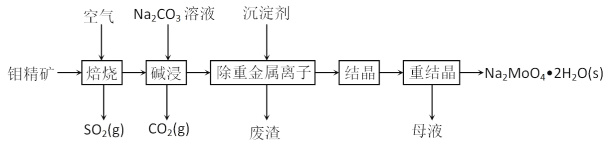
�ش��������⣺
��1����߱���Ч�ʵķ�����_______________________����дһ�֣�
��2����������ʱMoS2ת��ΪMoO3���÷�Ӧ���̵Ļ�ѧ����ʽΪ____________________������������__________________
��3���������ʱ����CO2�ĵ���ʽΪ______________�������ʱ���⻯���������Ҫ��Ӧ�����ӷ���ʽΪ____________________________��
��4���������ؽ���������ʱ����ij�����ΪNa2S��������ɷֵĻ�ѧʽΪ____________________��
��5���⾫�������������£�����NaNO3��Һ��Ҳ�����Ʊ������ƣ�ͬʱ��SO42-���ɣ��÷�Ӧ�����ӷ���ʽΪ____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳ���ˮ�ܿ�����Ҫ�ɷ�ΪCo2O3����������Fe2O3�� Al2O3��MgO��CaO�������� �Ʊ��ܵ���������Ʊ���������������
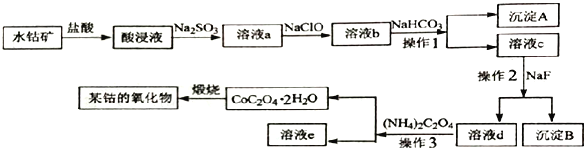
�ش�����������
��1���ڼ�����������������ʱ�����������������ʵķ�����______________����дһ������
��2��������������Na2SO3���ܵĴ�����ʽΪCo2+��д������Co2+��Ӧ�����ӷ���ʽ_______________________________��
��3����Һa�м���NaClO������Ϊ_______________________________��
��4������A �ijɷ�Ϊ__________________������2��������___________________��
��5����֪: Ksp (CaF2)=5.3��10-9��Ksp(MgF2)=5.2��10-12��������Һc �м���NaF��Һ����Mg2+ǡ�ó�����ȫ����Һ��c(Mg2+)=1.0��10-5moI/L����ʱ��Һ��c(Ca2+)������_________mol��L-1��
��6���ڿ���������CoC2O4 �����ܵ�ij���������CO2����ó�����պ��������Ϊ12.05 g��CO2�����Ϊ6.72 L����״��������˷�Ӧ�Ļ�ѧ����ʽΪ_______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com