
����Ŀ��д�����л�ѧ����ʽ�����ӷ���ʽ��
��1������ˮ��Ӧ�����ӷ���ʽ��___��
��2����������ͭ��Һ��Ӧ�����ӷ���ʽ��___��
��3�����������������̼��Ӧ�Ļ�ѧ����ʽ��___��
��4�������������������Ӧ�Ļ�ѧ����ʽ��___��
��5��þ�ڵ����е�ȼþ���Ļ�ѧ����ʽ��___��
��6��þ�ڶ�����̼�е�ȼþ���Ļ�ѧ����ʽ��___��
��7����������������Һ�ķ�Ӧ�����ӷ���ʽ��___��
��8��������������������Һ��Ӧ�����ӷ���ʽ��___��
��9����������������������Һ��Ӧ�����ӷ���ʽ��___��
��10������������������Ӧ�Ļ�ѧ����ʽ��___��
��11��ͭ���Ȼ�����Һ��Ӧ�����ӷ���ʽ��___��
���𰸡�2Na+2H2O=2Na++2OH�D+H2�� 2Na+Cu2++2H2O==Cu(OH)2��+H2��+2Na+ 2Na2O2+2CO2==2Na2CO3+O2 Na2O2+SO2==Na2SO4 3Mg+N2![]() Mg3N2 2Mg+CO2
Mg3N2 2Mg+CO2![]() 2MgO+C 2Al+2OH�C+2H2O=2AlO2�C+3H2�� Al2O3+2OH-=2AlO2-+H2O Al(OH)3+OH-=AlO2-+2H2O 8Al+3Fe3O4
2MgO+C 2Al+2OH�C+2H2O=2AlO2�C+3H2�� Al2O3+2OH-=2AlO2-+H2O Al(OH)3+OH-=AlO2-+2H2O 8Al+3Fe3O4![]() 4Al2O3+9Fe 2Fe3++Cu��2Fe2++ Cu2+
4Al2O3+9Fe 2Fe3++Cu��2Fe2++ Cu2+
��������
��1������ˮ��Ӧ�����ӷ���ʽ��2Na+2H2O=2Na++2OH-+H2����
��2����������ͭ��Һ��Ӧ�����ӷ���ʽ��2Na+Cu2++2H2O==Cu(OH)2��+H2��+2Na+��
��3�����������������̼��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2==2Na2CO3+O2��
��4�������������������Ӧ�Ļ�ѧ����ʽ��Na2O2+SO2==Na2SO4��
��5��þ�ڵ�����ȼ�յĻ�ѧ����ʽ��3Mg+N2![]() Mg3N2��
Mg3N2��
��6��þ�ڶ�����̼��ȼ�յĻ�ѧ����ʽ��2Mg+CO2![]() 2MgO+C��
2MgO+C��
��7����������������Һ�ķ�Ӧ�����ӷ���ʽ��2Al+2OH�C+2H2O=2AlO2�C+3H2����
��8��������������������Һ��Ӧ�����ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O��
��9����������������������Һ��Ӧ�����ӷ���ʽ��Al(OH)3+OH-=AlO2-+2H2O��
��10������������������Ӧ�Ļ�ѧ����ʽ��8Al+3Fe3O4![]() 4Al2O3+9Fe��
4Al2O3+9Fe��
��11��ͭ���Ȼ�����Һ��Ӧ�����ӷ���ʽ��2Fe3++Cu��2Fe2++ Cu2+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���ݻ�ΪV���ܱ��������м���һ�����ɻ����ĸ���(��Ȳ���)�������ֳ������֣�����߳���1molN2���ұ߳���һ������COʱ�����崦����ͼλ��(�����¶Ȳ���)������˵����ȷ���ǣ� ��
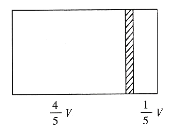
A.�ұ�����߷�����֮��Ϊ4��1
B.�Ҳ�CO������Ϊ5.6g
C.�Ҳ������ܶ�����ͬ�����������ܶȵ�14��
D.���ı��ұ�CO�ij�������ʹ���崦���������м䣬�����¶Ȳ��䣬��Ӧ����0.2molCO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʾ��ͼ���Ӧ�ķ�Ӧ�����ȷ������������

A. ��0.01 mol NaOH��0.01 mol Ba��OH��2�Ļ����Һ�л���ͨ��CO2
B. KHCO3��Һ����μ���Ba��OH��2��Һ
C. KAl��SO4��2��Һ����μ���Ba��OH��2��Һ
D. ���������������Ƶ�ƫ��������Һ�еμ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽��̼�����Ƶ����ȶ��ԣ��������������֤���������������̼�ķ�Ӧ��������װ��ͼ����ʵ�飬��Ҫ����ա�
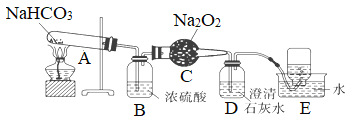
(1)A�з�Ӧ�Ļ�ѧ����ʽΪ________________________________��
(2)B��Ũ�����������__________________��
(3)C�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(4)D�з�Ӧ�����ӷ���ʽ��_______________________________��
(5)E���ռ���������Ҫ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ����������ʹ�õĺϽ�֮һ,��Ҫ��п��ͭ��ɡ��ش��������⣺
(1) ��̬пԭ�ӵĺ���۵����Ų�ʽΪ_______________,�������ڱ�__________��Ԫ�ء�����ռ������ܲ�ķ�����_______________ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ______________
(2)��һ������I1(Zn)________I1(Cu)(��������������С����)
(3)����ɫ ![]() ����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] 2+��
����ͭ��Һ�м����Թ����İ�ˮ,��Һ��Ϊ����ɫ[Cu(NH3)4] 2+��
������������SO42-��Ϊ�ȵ��������__________(�����)��
A.H2SO4 B.CO32- C.PO43- D.CCl4
��H2O��������ԭ�ӵ��ӻ�����Ϊ______��NH3���ӵĿռ乹��Ϊ________��
�����еļ��ǣ�H2O_______ NH3(��������������С����)��
��ͨ������ʵ�������֪����Cu2+�����λ����H2O_________NH3 (��������������С����)��
������Ӧ��ǰ���İ����飨BH3��NH3�������黥Ϊ�ȵ����塣д��BH3��NH3�Ľṹʽ���ṹ��������λ������![]() ����ʾ��_______________________
����ʾ��_______________________
(4)����Cu�����е�ԭ�Ӷѻ���ʽ��ͼ��ʾ,���ֶѻ���ʽ��Ϊ_____________��

(5)��Cu������ܶ�Ϊ��g/cm3,![]() ��ʾ�����ӵ�������ֵ,��ʽ��ʾCu���������������Cuԭ��֮��ľ���________nm(���ػ���)
��ʾ�����ӵ�������ֵ,��ʽ��ʾCu���������������Cuԭ��֮��ľ���________nm(���ػ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����N2��H2�������ֱ����ס��ҡ������������н��кϳɰ���Ӧ������һ��ʱ�������ʷ�Ӧ����Ϊ����(H2)��3 mol��L��1��min��1������(N2)��2 mol��L��1��min��1������(NH3)��1 mol��L��1��min��1�������ʱ�������������кϳɰ��ķ�Ӧ����(����)
A. �ף��ң��� B. �ף��ң��� C. �ң��ף��� D. �ף�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��50 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ��ͼʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
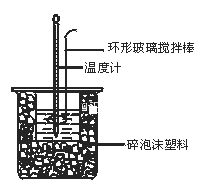
��1��ͼ�л��ν�����ܷ���ͭ����Ʒ����______��ԭ����____________
��2���ձ���������ֽ����������__________________________________
��3��ÿһ��ƽ��ʵ��������Ҫ�۲��¼�����¶���ֵ______
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ_______________���ƫ��ƫС������Ӱ�족����
��5��ʵ���и���60 mL 0.50 mol��L��1�����50 mL 0.55 mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ������к���____________�����ȡ�������ȡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��������һ�ַdz����õĽ�����ͨ������________�С�����Ͷ������ͭ��Һ�У�������Ӧ�����ӷ���ʽΪ_____________��_________________
(2)Na2O2����Ϊ��������еĹ��������乩��ʱ��Ӧ�Ļ�ѧ����ʽ�У�__________��_____
(3)��һ����Һ�����ܺ���Al3����Fe3����K����Mg2����Cu2���������е�һ�ֻ��֡��ּ���Na2O2��ĩ����ɫ��ζ������ų���ͬʱ������ɫ������������Һ�е�ˮ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ�����ƶϣ�

��ԭ��Һ��һ�����е�������__________________��
��һ�������е�������_________________��
�ۿ��ܺ���___________��Ϊ�˽�һ��ȷ�����ܺ��и����ӣ���������ɫ��Ӧ��ʵ�飬����ɫ�ܲ����۲쵽�Ļ������ɫΪ_______ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�����CaCl2��HCl�Ļ����Һ����μ���Ũ��Ϊ1 mol��L��1��Na2CO3��Һ����Ӧ�����м����Na2CO3��Һ���������������������������ϵ��ͼ��ʾ��
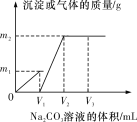
��֪ͼ��V1��V2��1��2��������V2 mL Na2CO3��Һʱ��������Һ��Ũ��Ϊ1 mol��L��1�����Ϊ200 mL����
(1)����V2 mL Na2CO3��Һʱ��������Һ��������________��
(2)ԭ�����Һ��CaCl2��HCl�����ʵ���֮��n(CaCl2)��n(HCl)��________��
(3)m1��________g��m2��________g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com