
����Ŀ���ҹ�����Ϊ�˼���ȼú��ɵĴ�����Ⱦ��������ȼ��ú�����˶��ĸĽ���
(1)Ϊ�˳�ȥú�еĺ�����ɲ���FeCl3������FeCl3��Һ��ϴú�ۣ��������·�Ӧ��FeS2��14FeCl3��8H2O=2FeSO4��13FeCl2��16HCl��Ϊ�˳������Fe2��������HCl��Ⱦ�������ù�ҵ����м���������Һ������������FeCl3�ķ�������д����һ�������йص����ӷ���ʽ��___________________________________��
(2)��һ�ַ����Dz��ù�������ȼú�м�����ʯ�ң�ʹ����ú��ȼ��ʱ���ɵ�SO2�����ݳ�������¯���У����û�ѧ����ʽ��ʾ��һ�����������̣�_______________________________��
(3)ij���в�����������������ú������ȼ�ϵ�����������������Ҫ�ɷ��DZ��飬д����ȼ�յĻ�ѧ����ʽ��________________________��
���𰸡�Fe��2H��=Fe2����H2��,2Fe2����Cl2=2Fe3����2Cl�� SO2��CaO![]() CaSO3��2CaSO3��O2
CaSO3��2CaSO3��O2![]() 2CaSO4 C3H8��5O2
2CaSO4 C3H8��5O2![]() 3CO2��4H2O
3CO2��4H2O
��������
(1)���빤ҵ����м������H��������HCl��Ⱦ����ͨ��Cl2������Fe2����
(2)������������ʯ�ҽ�SO2ת��ΪCaSO3��CaSO3�ڸ���ʱ������ΪCaSO4��
(3)C3H8ȼ�����ɶ�����̼��ˮ��
(1)���빤ҵ����м������H��������HCl��Ⱦ����ͨ��Cl2������Fe2����������Ӧ�����ӷ���ʽ�ֱ�ΪFe��2H��=Fe2����H2����2Fe2����Cl2=2Fe3����2Cl����
(2)������������ʯ�ҽ�SO2ת��ΪCaSO3��CaSO3�ڸ���ʱ������ΪCaSO4���û�ѧ����ʽ��ʾ��һ��������ΪSO2��CaO![]() CaSO3��2CaSO3��O2
CaSO3��2CaSO3��O2![]() 2CaSO4��
2CaSO4��
(3)C3H8ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪC3H8��5O2![]() 3CO2��4H2O��
3CO2��4H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬѧ�Ƕ�������������������Ȥ���Ӳο��������ҵ�����������������ӡ�øߵ������������۵Ĺ������̼�ͼ��
[��������]
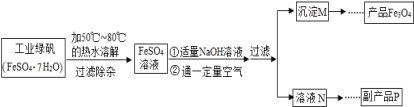
[��������]
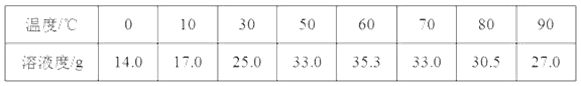
����һ�����������ڲ�ͬ�¶��µ��ܽ�����±���ʾ��
���϶�������������������������Ӧԭ��Ϊ4Fe(OH)2��2H2O��O2=4Fe(OH)3
����������������������ķ�Ӧԭ��ΪFe(OH)2��2Fe(OH)3![]() Fe3O4��4H2O
Fe3O4��4H2O
[��������]
(1)�ܽ�ʱ����50����80�����ˮĿ����______________��
(2)д��������������������Һʱ������Ӧ�Ļ�ѧ����ʽ_____________��
(3)���������У�Ҫ������ͨһ������������Ŀ����____________��
(4)Ҫʹ�����������IJ�����ߣ������������в������������������������������������Ϊ___��
(5)����ƷP�������ƣ�����ҺN��øø���Ʒ�IJ���˳����b��______��______��d��
a������b������Ũ��c����ȴ�ᾧd�������������ȫʧȥ�ᾧˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����X��Y��Z���ֶ�����Ԫ�أ�X����̬�⻯�ﻯѧʽΪH2X�����⻯���ʽ����X������������ʽ��֮��Ϊ17�� 40��Xԭ�Ӻ�������������������ȣ�Y��X�����γ����ӻ�����Y2X��Y�������ӵ��Ӳ�ṹ��Ne��ͬ������Xͬ���ڣ�����̬������˫ԭ�ӷ��ӣ���ԭ�ӹ���1�Ե��ӡ��Իش�
(1)д����Ԫ�ط��ţ�X__________��Y_________��Z__________��
(2)X���ӵĽṹ��ͼΪ____________________��X��Y�γɵ����ӻ�����Ļ�ѧʽΪ___________________��Z�����γɵĻ�����Ļ�ѧʽΪ________________��
(3)Y�����ڿ�����ȼ�յĻ�ѧ����ʽΪ __________________________________����������ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ���H(![]() )��һ�־�������ά���㷺���ڸ���ɲ��Ƭ����ϳ�·�����£�
)��һ�־�������ά���㷺���ڸ���ɲ��Ƭ����ϳ�·�����£�
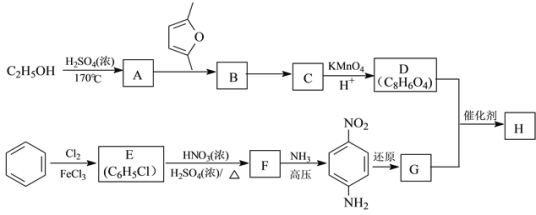
��֪����C��D��G��Ϊ�����廯��������о�ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�
��Diels-Alder��Ӧ��![]()
(1)����A�ķ�Ӧ������___________��F�����������ŵĽṹ��ʽΪ______________��
(2)B�Ľṹ��ʽ��___________����B��C���ķ�Ӧ�У���C�⣬����һ�ֲ���������______��
(3)D+G��H�Ļ�ѧ����ʽ��_____________________________________________��
(4)Q��D��ͬϵ���Է���������D��14����Q���ܵĽṹ��______�֣����к˴Ź���������5��壬�ҷ������Ϊ1:2:2:2:1�Ľṹ��ʽΪ_____________________��
(5)��֪����Ȳ��1��3-����ϩҲ�ܷ���Diels-Alder��Ӧ������1��3-����ϩ����ȲΪԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�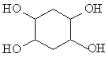 ��д���ϳ�·��______________��(�ϳ�·������ͼʾ��:H2C=CH2
��д���ϳ�·��______________��(�ϳ�·������ͼʾ��:H2C=CH2![]() CH3CH2OH
CH3CH2OH![]() CH3COOC2H5)��
CH3COOC2H5)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ռ�������ˮ�����ξ��ơ���һ�ξ�����Ҫ���ó�������ȥ����ˮ��Ca2+��Mg2+��Fe3+��SO![]() �����ӣ��������£�
�����ӣ��������£�
��. �����ˮ�м������BaCl2��Һ�����ˣ�
��. ��������Һ�м������Na2CO3��Һ�����ˣ�
��. ��Һ���������pH�����һ�ξ�����ˮ��
(1)��������ȥ��������______��
(2)�������������ɵIJ��ֳ��������ܽ��(20��/g)���±���
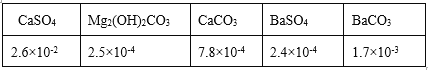
�ټ��Fe3+�Ƿ�����ķ�����______��
�ڹ�����ѡ��BaCl2����ѡ��CaCl2�����ñ������ݽ���ԭ��______��
�۳�ȥMg2+�����ӷ���ʽ��______��
�ܼ��Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ�ԭ����_____��
(3)�ڶ��ξ���Ҫ��ȥ����I-��IO![]() ��NH
��NH![]() ��Ca2+��Mg2+������ʾ�����£�
��Ca2+��Mg2+������ʾ�����£�
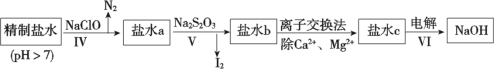
�� ��������ȥ��������______��
�� ��ˮb�к���SO![]() ��Na2S2O3��IO
��Na2S2O3��IO![]() ��ԭΪI2�����ӷ���ʽ��________ ��
��ԭΪI2�����ӷ���ʽ��________ ��
�� ����VI�У��ڵ��۵�����������Ӧ�ĵ缫����ʽ�ǣ�_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����[aX��bY]Ϊa��X����b��Y����ɵ�һ���������壬NAΪ�����ӵ�������ֵ������˵������ȷ����
A.H2(g)��![]() O2(g)=H2O(l)����H����286 kJ��mol1����ÿ1 mol [H2(g)��
O2(g)=H2O(l)����H����286 kJ��mol1����ÿ1 mol [H2(g)��![]() O2(g)]����1 mol [H2O(l)]����286 kJ
O2(g)]����1 mol [H2O(l)]����286 kJ
B.Cr2O72-��ne��14H+=2Cr3+��7H2O����ÿ����1 mol Cr3+ת�Ƶ�����Ϊ3NA
C.Al3+��4OH=[Al(OH)4]��˵��1 mol Al(OH)3�����H+��ΪNA
D.1 mol CO2��NaOH��Һ��ȫ��Ӧ����n(CO32-)��n(HCO3-)��n(H2CO3)��1 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ��ʾ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�Ӧ(N2��O2����I2��Ӧ)ΪSO2��I2��2H2O=H2SO4��2HI��
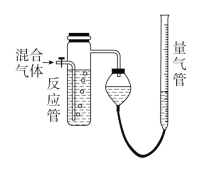
(1)���������뷴Ӧ�ܺ������������ӵ�ˮ���������________________(������ķ���ʽ)�������
(2)��Ӧ���ڵ���Һ��ɫ��ʧ��û�м�ʱֹͣͨ�������õ�SO2����________(����ƫ������ƫ������������Ӱ����)��
(3)��Ӧ���ڵĵ�ĵ�����ҺҲ������________(����������)���档
(4)������Һ���ΪVa mL��Ũ��Ϊc mol��L��1��N2��O2�����ΪVb mL(������Ϊ��״���µ����)����c��Va��Vb��ʾSO2������ٷֺ�����____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����NaOH��Һ��������Һ��
������100mL 1.0mol��L-1 NaOH��Һ

��1����ͼ��ʾ��������E������Ϊ___��������Һ�϶�����Ҫ��������___������ţ����������ӵIJ���������___�����������ƣ���
��2��������NaOH��Һʱ
�ٸ��ݼ�����������ƽ��ȡNaOH������Ϊ___g��
�����в�����������ҺŨ�ȵ�Ӱ����(����ƫ������ƫ��������Ӱ����)��
���� | Ũ��Ӱ�� |
����ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ��� | ___ |
û��ϴ���ձ��Ͳ����� | ___ |
����ʱ�����Ӷ��� | ___ |
����Eδ�����������ˮ | ___ |
������100mL 0.5mol��L-1 ������Һ
����������Ϊ98%���ܶ�Ϊ1.84g��cm-3��Ũ���������Ƹ���Һ����Ũ�������ʵ���Ũ��Ϊ___mol��L-1������Ũ��������Ϊ___mL (����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ȤС����ʵ���ҽ����������������ʵ�������ͼ��
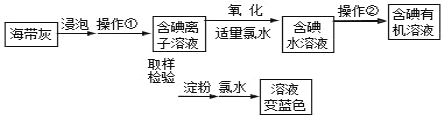
��1�������ٵ�������_____�������ڵ���Ҫ������_____��
��2��̽���쳣��ȡ������ʱ������ͬѧû�й۲쵽��Һ����ɫ�����Ǽ���ԭ������Ǽ������ˮ�����������ˢ�I2���ڵ��ۣ���I2�͵��ۡ�������û�б���ɫ����Һ�У��μ�_____ (ѡ�������Ȼ�̼������ˮ����������Һ��)��������_____������֤���������ȷ��ͬʱ�ų�����ڢۡ���ͬʱ�ų�����ڢ۵�ԭ����_____��
��3��̽�������ԣ���ʢ��FeCl3��Һ���Թ��У����뼸��KI��Һ������Ӧ�����Һ���ȵ�����֧�Թܣ��Թ�a�м���1mL�����ã�����_____ (��ʵ������)��֤����I2���ڣ��Թ�b�е���KSCN��Һ����Һ��Ѫ��ɫ��֤����_____���ڡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com