
����Ŀ��I.��������9�����ʣ����������ڶ�������ϡ�����ͭ�����Ȼ������壻���������ع��壻���������Һ����ƾ�(C2H5OH)���������ƹ��壬�뽫�������ʰ�Ҫ�������������(����Ӧ��ѧʽ���)��
��1�������������������___________�����ڵ���ʵ���_______________________��
II.ij����С�����Fe(OH)3������Ʊ�ʵ�鲢������������ʡ�
��2����������FeCl3��Һ�ֱ��������Һ���У����γɽ������___________��
A.��ˮ B.��ˮ C.NaOHŨ��Һ D.NaClŨ��Һ
��3��д���Ʊ�Fe(OH)3����Ļ�ѧ����ʽ��__________________________��
��4���������������ȶ����ڵ���Ҫԭ����______________(ѡ�����)��
A.������ֱ��С��1nm B.�������������
C.�������������˶� D.������������ֽ
���𰸡�SO2 BaCl2��KOH��KNO3 B FeCl3+3H2O![]() Fe(OH)3(����)+3HCl B
Fe(OH)3(����)+3HCl B
��������
��1�������������ֻ�ж�������������ƣ���������ˮ��Ӧ�����������ƣ�����������Ϊ���������������������ˮ��Ӧ�����������ᣬ�ɴ˿�ȷ���������������ʶ��Ǵ����ͨ��Ϊ�ᡢ��Ρ������������ˮ���ɴ˿�ȷ������ʡ�
��2����ȡ������������ʱ��������FeCl3��Һ�����ˮ�С�
��3���Ʊ�Fe(OH)3����ķ�Ӧ����FeCl3��ˮ�ⷴӦ��
��4��������������֮�����ȶ����ڣ���Ҫ����Ϊ����������ͬ������ɡ�
��1�������������ֻ�ж�������������ƣ���������ˮ��Ӧ�����������ƣ�����������Ϊ���������������������ˮ��Ӧ�����������ᣬ��������������ΪSO2������ʶ��Ǵ����ͨ��Ϊ�ᡢ��Ρ������������ˮ��������ΪBaCl2��KOH��KNO3����Ϊ��SO2��BaCl2��KOH��KNO3��
��2����ȡ������������ʱ��������FeCl3��Һ�����ˮ�С���Ϊ��B��
��3���Ʊ�Fe(OH)3����ķ�Ӧ������FeCl3��ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪFeCl3+3H2O![]() Fe(OH)3(����)+3HCl����Ϊ��FeCl3+3H2O
Fe(OH)3(����)+3HCl������FeCl3+3H2O![]() Fe(OH)3(����)+3HCl��
Fe(OH)3(����)+3HCl��
��4��������������֮�������ȶ����ڣ���Ҫ����Ϊ����������ͬ������ɡ���Ϊ��B��


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ļס��ҡ������ֽ������ֱ�����������������������ͬ��ϡ������ȫ��Ӧ�������ɣ�2�۵������Σ����������������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����Ƚ����д�С��ϵ��
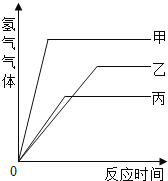
(1)���ֽ����Ļ����ǿ������˳��Ϊ��________________________��
(2)���ֽ�����Ӧʱ����������������ٵ����˳��Ϊ��_____________��
(3)���ֽ��������ԭ�������ɴ�С��˳��Ϊ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ����Ч������ҵ�������ŷŵĵ�������(NOx)��SO2�ȣ����ٴ�����Ⱦ����ѧ�Ҳ��϶���ط�Ӧ�����о����ԡ�
��1����ÿ��ȫ������ȼú8�ڶ֣�ú�к�������������2%�ƣ����������������������_______�ڶ�SO2�ŷŵ������У�����Sȫ��ת��ΪSO2����
��2�����������У�������SO2����_______������ţ���
a����ˮ b������KMnO4��Һ c����ʯ�� d��Na2CO3��Һ
��3��ij��������(NH4)2SO3��NH4HSO3�Ļ����ҺA���շ����е�SO2���Ʊ�(NH4)2SO3��H2O���������£�
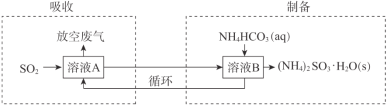
�������ա������У���Һ��(NH4)2SO3��NH4HSO3���ʵ���֮�ȱ�_____�����С������
�����Ʊ��������У���ҺB�з�����Ӧ�Ļ�ѧ����ʽ�� _____________________��
�������Ʒ(NH4)2SO3��H2O�к�������SO42-�ķ������£�ȡ������Ʒ��ˮ�ܽ⣬����ʵ�����������������___________________________________��
��4��ѡ���Դ���ԭ����(SCR)��Ŀǰ��Ϊ��������������������䷴Ӧԭ����ҪΪ��4NH3(g)��4NO(g)��O2(g) ![]() 4N2(g)��6H2O(g) ��H= -1627 kJmol-1
4N2(g)��6H2O(g) ��H= -1627 kJmol-1
����NO������NH3�ͱ�O2������NH3�����ʵ���֮��Ϊ________________��
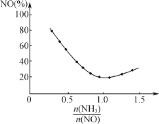
�ڰ�����![]() ��ֱ��Ӱ��÷�������������ͼΪ350 ��ʱֻ�ı䰱����Ͷ������NO�İٷֺ����백���ȵĹ�ϵͼ����
��ֱ��Ӱ��÷�������������ͼΪ350 ��ʱֻ�ı䰱����Ͷ������NO�İٷֺ����백���ȵĹ�ϵͼ����![]() >1.0ʱ��������NO��������������Ҫԭ����__________________��
>1.0ʱ��������NO��������������Ҫԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ᴿ��������ʵķ����У�A����ȡ��Һ B�����ȷֽ� C�������ᾧ D����Һ E������ F������ G�������ȣ��뽫�ᴿ������������ں�������ϡ�
��1�����뱥��ʳ��ˮ����ɳ�Ļ����___��
��2������Fe(OH)3����![]() ����NaCl��Һ
����NaCl��Һ![]() ___��
___��
��3���������ܵ�CCl4(�е�Ϊ76.75��)�ͼױ�(110.6��)�Ļ����___��
��4���ӵ�ˮ����ȡ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧ��Ӧԭ��֪ʶ�о��������CO��SO2����Ⱦ������Ҫ���塣
��1����CO���Ժϳɼ״�����֪��
CH3OH(g)��3/2O2(g)===CO2(g)��2H2O(l) ��H����764.5 kJ��mol��1
CO(g)��1/2O2(g)===CO2(g) ��H����283.0 kJ��mol��1
H2(g)��1/2O2(g)===H2O(l) ��H����285.8 kJ��mol��1
��CO(g)��2H2(g) ![]() CH3OH(g) ��H��______kJ��mol��1��
CH3OH(g) ��H��______kJ��mol��1��
��2�����д�ʩ���ܹ����������ϳɼ״���Ӧ���ʵ���________(��д���)��
a��ʹ�ø�Ч���� b�����ͷ�Ӧ�¶�
c��������ϵѹǿ d�����Ͻ�CH3OH�ӷ�Ӧ������з������
��3����һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ
����ͼ��ʾ��
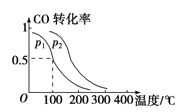
��p1________p2(����ڡ�����С�ڡ����ڡ�)��
��100 ��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K��________��
���������������������£�������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����________(���������С�����䡱)��
��4��ij����С����SO2Ϊԭ����ȡ���ᡣ
������ԭ���ԭ������SO2��O2��H2O���Ʊ����ᣬ�õ���ö�ײ������缫�������������壬ͬʱҲ��ʹ������������Һ��ֽӴ�����д���õ�ظ����ĵ缫��Ӧʽ��_____________________��
����Na2SO3��Һ�������SO2��NaHSO3��Һ��Ȼ�������Һ���Ƶ����ᡣ���ԭ��ʾ��ͼ���¡���д����ʼʱ������Ӧ�ĵ缫��Ӧʽ��_________________��
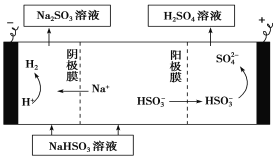
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������̵���Ҫ������ͳ��õ��������������ǹ�ҵ�������̿��Ʊ�������ص�һ�ֹ������̡�
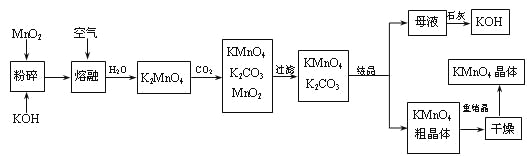
��1��KMnO4ϡ��Һ��һ�ֳ��õ�������������������������___������ţ��������ơ�
A��75%�ƾ� B��˫��ˮ C������ D��84����Һ��NaClO��Һ��
��2��д��MnO2��KOH�����ڻ������ͨ�����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽ��
___________________________________________________________________��
��3����K2MnO4��Һ��ͨ��CO2���Ʊ�KMnO4���÷�Ӧ�еĻ�ԭ���ǣ�_______��
��4�����������п���ѭ��ʹ�õ�������ʯ�ҡ�������̼��___��___��д��ѧʽ����
��5��������������ѭ�����Ʊ������е���ʧ����1 mol MnO2���Ƶ�_____mol KMnO4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ��ƺ������Ϊԭ���Ʊ��Ȼ�識�����Ʒ������,������������:
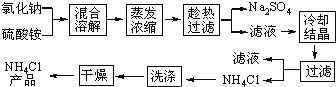
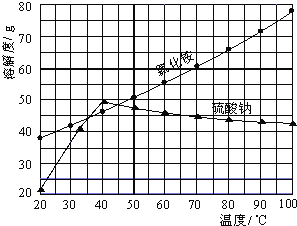
�Ȼ�狀������Ƶ��ܽ�����¶ȱ仯����ͼ��ʾ���ش��������⣺
��1�����Ʊ�10.7gNH4Cl����������NaCl_________g��
��2��ʵ���ҽ�������Ũ���õ�����Ҫ������________���ձ������������ƾ��Ƶȡ�
��3������ȴ�ᾧ�������У�����NH4Cl����ĺ����¶�Ϊ_________��
��4�����������Լ������NH4Cl��Ʒ�Ƿ��ķ�����������_________________��
��5����NH4Cl��Ʒ�к������������ʣ���һ���ᴿ��Ʒ�ķ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��M��Q��R��Ϊǰ20��Ԫ�أ���ԭ�Ӱ뾶����Ҫ���ϼ۵Ĺ�ϵ��ͼ��ʾ������˵��������

A. Qλ�ڵ�������IA��
B. X��Y��Z����Ԫ����ɵĻ�����������λ��
C. �����Ӱ뾶��M->Q+>R2+
D. Z��M������������Ӧˮ�����Ϊǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ơ���������������Ҫ�Ľ�������ش�
��1�����ֽ�����ͬŨ�����ᷴӦ����Ӧ��������___(��ѡ��)��
a���� b���� c����
��2����������ˮ������Ӧ�����ӷ���ʽΪ___������ѡ�
a��2Na +2H2O=2NaOH+H2��
b��2Na+2H2O=2Na++2OH-+H2��
c��2Na +2H2O=2Na++2OH-+O2��
��3���ɹ۲쵽��ʵ��������ȷ����__(��ѡ��)��
a���Ƴ���ˮ�� b�����۳�С�� c��С���Ĵ��ζ�
��4����дa��b��c��ѧʽ����д�ٵĻ�ѧ����ʽ��a��b��c��Ϊ��Al�������
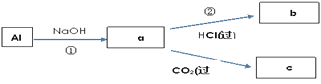
a��___��b��___��c��___��
��____________��
��5��Fe��Cl2��һ�������·�Ӧ�����ò���Ϊ___��
��6�����ò�������ˮ�����Һ����װ����֧�Թ��С���ش�
a����������һ֧�Թ��еμ�KSCN��Һ������Һ���__ɫ��
b������һ֧�Թ��еμ�NaOH��Һ������__ɫ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com