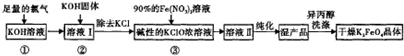
���
�⣺��1��A��ʵ���Ҹɷ��Ʊ����ü�KOH������������������A����
B���������������������������ز���Ӧ����B��ȷ��
C�������������ܺ��������Ʒ�Ӧ����ƫ�����ƣ���C����
D���մ��������ж������裬���������ܺ��������Ʒ�Ӧ����D����
��ѡB��
��2������Ŀ��Ϣ��������ԭ��Ӧ�л��ϼ۷����仯ȷ�������У�KClO
3��KCl��H
2O��Cl
2�������������ǻ�ԭ�������ݵ��ӵ�ʧ�غ�������غ�����ƽ���ɵ�6KOH+3Cl
2KClO
3+5KCl+3H
2O��
�ʴ�Ϊ��6KOH+3Cl
2KClO
3+5KCl+3H
2O��
��3��A��KClO
3ת��Ϊ KClO�����ϼ�ֻ����������ʵ�֣���A����
B���ڢ۲���Ҫ�������������Լ�Ҫ������Ϊ��һ����Ӧ�ṩ���ԵĻ�������B��ȷ��
C��KOH������Һ���ȣ��¶Ƚϸ�ʱKOH��Cl
2��Ӧ���ɵ���KClO
3��������KClO����C����
D������������������ͼ���ڢٲ���������������KOH�����������Ӧ����KClO����D��ȷ��
��ѡBD��
��4������Ŀ��Ϣ��������ԭ��Ӧ�л��ϼ۷����仯ȷ���ҳ���Ӧ�Fe
3+��ClO
-�������FeO
42-��Cl
-�����ݵ��ӵ�ʧ�غ�������غ�����ƽ���ɵ�2Fe
3++3ClO
-+10OH
-=2FeO
42-+3Cl
-+5H
2O��
�ʴ�Ϊ��2Fe
3++3ClO
-+10OH
-=2FeO
42-+3Cl
-+5H
2O��
��5�������������ˮϴ�Ӳ�Ʒ�������Ʒ�ܽ���ʧ��ͬʱ������ӷ������ڸ��
�ʴ�Ϊ������K
2FeO
4����ϴ��ʱ��Ʒ����ʧ�Ҳ�Ʒ���
��6����ֻҪ�������һ�ε�ϴ������Cl
-������֤��K
2FeO
4�����Ѿ�ϴ�Ӹɾ���
�ʴ�Ϊ�����Թ�ȡ�������һ�ε�ϴ��Һ��������������Һ���ް�ɫ�������ѱ�ϴ����
��7�����ݵ��ӵ�ʧ�غ�������غ�����ƽ���ɵ�4FeO
42-+10H
2O�T4Fe��OH��
3�����壩+3O
2��+8OH
-��
�ʴ�Ϊ��4FeO
42-+10H
2O�T4Fe��OH��
3�����壩+3O
2��+8OH
-



 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
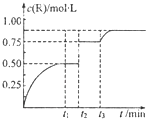 һ���¶��£���1molN�������2L�����ܱ������У�������Ӧ��M��g��+N��g��?xR��g��+Q��s����t1ʱ�ﵽƽ�⣮��t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ���������������������R��Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������
һ���¶��£���1molN�������2L�����ܱ������У�������Ӧ��M��g��+N��g��?xR��g��+Q��s����t1ʱ�ﵽƽ�⣮��t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ���������������������R��Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������