
����Ŀ����������ʮ�����ʣ���H2 ���� �۴��� ��CO2 ��H2SO4 ��Ba(OH)2���� �߰�ˮ ��ϡ���������Al2(SO4)3 ��NaHSO4��
(1)�����ʵķ������д����Ŀհ״���
���ڷǵ���ʵ���__________�����ڵ���ʵ���__________���ܵ������____________��
(2)����ʮ������������������֮��ɷ������ӷ�Ӧ��H++OH===H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ_____________________________��
(3)д���ۺ͢߷�Ӧ�����ӷ���ʽΪ__________________��34.2 g ������ˮ���250 mL��Һ��SO42-�����ʵ���Ũ��Ϊ_________________��
(4)�����Ģ�ͨ�����Һ�з�Ӧ�����ӷ���ʽΪ______________________��
(5)�������Һ�������Һ������Һ�е�Ba2+����ǡ����ȫ����ʱ��Ӧ�����ӷ���ʽΪ__________��
���𰸡��ܢۢݢޢ��ڢߢ��Ba(OH)2+2HNO3=Ba(NO3)2+2H2OCH3COOH+NH3��H2O=CH3COO+NH4++H2O1.20 mol/LCO2+OH=HCO3-2H++SO42-+Ba2++2OH= BaSO4��+2H2O
��������
(1). CO2 ����Һ���������ܵ��룬���ڷǵ���ʣ����ᡢ���������ᣬBa(OH)2���ڼAl2(SO4)3��NaHSO4�����Σ���ˮ��Һ�ж����Ե��������ʹ��Һ���е����ԣ����ڵ���ʣ����ǽ��������Ե��磬��ϡ������Һ�У��������������Ӻ���������ӣ����Ե��磬�ڰ�ˮ��Һ�У�һˮ�ϰ������笠����Ӻ����������ӣ����Ե��磬����Al2(SO4)3����������Ӻ���������ӣ����Ե��磬�ʴ�Ϊ���ܣ��ۢݢޢ�⣻�ڢߢ��
(2.)����������������Ӧ��ʵ���������������������ӷ�Ӧ����ˮ�����������ӷ���ʽH++OH=H2O��ʾ����Ӧ��Ӧ����ʽΪ��Ba(OH)2+2HNO3=Ba(NO3)2+2H2O���ʴ�Ϊ��Ba(OH)2+2HNO3=Ba(NO3)2+2H2O��
(3). �����һˮ�ϰ���ˮ��Һ�ж�������ȫ���룬���Դ���Ͱ�ˮ��Ӧ�����ӷ���ʽΪCH3COOH+NH3H2O=CH3COO+NH4++H2O��34.2 g �����������ʵ���Ϊ![]() =0.10mol�����������Ļ�ѧʽ��֪����������ӵ����ʵ���Ϊ0.30mol������ˮ���250 mL��Һ��SO42-�����ʵ���Ũ��Ϊc(SO42-)=
=0.10mol�����������Ļ�ѧʽ��֪����������ӵ����ʵ���Ϊ0.30mol������ˮ���250 mL��Һ��SO42-�����ʵ���Ũ��Ϊc(SO42-)=![]() =1.20mol/L���ʴ�Ϊ��CH3COOH+NH3H2O=CH3COO+NH4++H2O��1.20mol/L��
=1.20mol/L���ʴ�Ϊ��CH3COOH+NH3H2O=CH3COO+NH4++H2O��1.20mol/L��
(4).Ba(OH)2��Һ�������CO2��Ӧ����̼���Ⱶ���÷�Ӧ�����ӷ���ʽΪ��OH+CO2= HCO3-���ʴ�Ϊ��OH+CO2= HCO3-��
(5).���������Ƶ���Һ����Ba(OH)2��Һ������Һ�е�Ba2+����ǡ����ȫ����ʱ��Ba(OH)2��NaHSO4�����ʵ���֮��Ϊ1:2���÷�Ӧ�����ӷ���ʽΪ��2H++SO42+Ba2++2OH=BaSO4��+2H2O���ʴ�Ϊ��2H++SO42+Ba2++2OH=BaSO4��+2H2O��


 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й�ϵ����˵�����淴ӦN2+3H2 ![]() 2NH3�Ѵﵽƽ��״̬����
2NH3�Ѵﵽƽ��״̬����
A. ����(N2)=����(NH3) B. ����(N2)=3����(H2)
C. 3����(N2)=����(H2) D. 2����(H2)=3����(NH3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ����1.0L w molL-1CH3COOH��Һ��0.1molNaOH�����ϣ���ַ�Ӧ��Ȼ������Һ�м���CH3COOH�� CH3COONa���壨������Һ������¶ȱ仯������ҺpH�仯��ͼ��ʾ������������ȷ����

A. a��b��c��Ӧ����Һ�У�ˮ�ĵ���̶��ɴ�С��˳���ǣ�c>a>b
B. w��0.1
C. ��b��a�Ĺ����У�[c(Na+)��c(OH-)]/c(CH3COO-)����
D. 25��ʱ��CH3COOH�ĵ���ƽ�ⳣ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�������ȫ��ȷ�ĸ�������( )
�����Թܼмг��Թ�ʱ,�Թܼд��Թܵײ�������,�����Թܵ����ϲ�
�ڸ�ʢװҺ����������Թ��ݻ�����֮һ���Թܼ��� �۱��ӿ���������ȥ���������ζ �ܽ��Թ�ƽ��,��ֽ�۽������ĩ�����Թܵײ�,Ȼ�������Թܢ�����ʵ��ʱ�¶ȼ�Ӧ����Һ�����²���Һ���¶� ����ƿ��������,��������ƿ�� ��������ǯ��ȡ���Ⱥ�������� ������ʵ�����ʱ����������ƿ��δ�ӷ�ʯ��ӦѸ�ٲ��ӷ�ֹ���� ��ϡ��Ũ����ʱ,��ˮ����ʢ��Ũ�������Ͳ�� ������ʵ���ȵ�ȼ�ƾ��ƺ���ͨ����ˮ
A.3��B.4��C.5��D.6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к͵ζ����ⶨij�ռ�Ĵ��ȣ��Ը���ʵ��ش�
��1����ȡ4.1g�ռ���Ʒ������Ʒ���250mL����Һ����Ҫ����Ҫ�����������ձ������������__________________ ��______________________��
��2��ȡ10.00mL����Һ����___________________��ȡ��
��3����0.2010mol��L-1������ζ������ռ���Һ���Է�̪Ϊָʾ�����ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע��_____________��ֱ������______________________________�����жϴﵽ�ζ��յ㡣
��4�������������ݣ���������ռ���Һ��Ũ��Ϊ��_____________________�����������λ��Ч���֣�����Ʒ�ռ����������Ϊ________________�����������λ��Ч���֣���(�����ռ��в��������ᷴӦ������)
�ζ����� | ����Һ��� (mL) | ���������(mL) | |
�ζ�ǰ����(mL) | �ζ������(mL) | ||
��һ�� | 10.00 | 0.50 | 20.40 |
�ڶ��� | 10.00 | 4.00 | 24.10 |
��5���ζ����������������ʹ�ⶨ���ƫ�ߵ���_____________________________����ţ���
����ʽ�ζ�����ˮϴ���װҺ����еζ����ڼ�ʽ�ζ���ˮϴ��������ȡ����Һ������ƿ������ˮϴ�Ӻ����ô���Һ��ϴ���ܵζ������ϸ��������Һ������ƿ�ڶ���δҡ��ϴ�£��������ڵζ�ʱ������ƿ�⣻�μ����ᣬ��Һ��ɫ��ȥ�����������ָֻ���ɫ���ߵζ�ǰ����ʽ�ζ��������ݣ��ζ�����ʧ�����¼��ʼ���ʱ�����Ӷ������յ�ʱ���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ����ֺ���������ˮ�е��ܽ������±���ʾ��
���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
�ù��յ���Ҫ�������¡�

��ش��������⣺
��1����д������Na2SO3��Һ������Ӧ�����ӷ���ʽ_________��
��2����������ʹ�õĴ�������ý(V2O5)�ܼӿ���������������ʣ��˹����в�����һ�������м��壨����ͼ��������a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ_________________��______________��
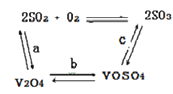
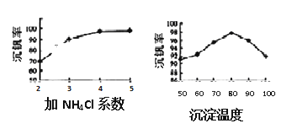
��3���ù����г������ǻ��շ��Ĺؼ�֮һ�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ����NH4Cl������������Һ��V2O5�������ȣ����¶ȡ�������ͼ�Խ�������Ȼ��ϵ��Ϊ_________�������¶ȵķ���Ϊ_________________��
��4������Һ1����Һ2��Ϻ����������������Ԫ�ر���ԭΪ��ͼۣ��䷴Ӧ�����ӷ�Ӧ����ʽΪ___��
��5���������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ仯��������ͼ��ʾ����NH4VO3�ڷֽ������_________��

A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3 B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O
C��ͬʱ�ֽ�ʧȥH2O��NH3D��ͬʱ�ֽ�ʧȥH2��N2��H2O
��6��ȫ����صĵ������ҺΪVOSO4��Һ����صĹ���ԭ��ΪVO2+ + V2++2H+ ![]() VO2+ +H2O +V3+����س��ʱ�����ĵ缫��ӦʽΪ___________��
VO2+ +H2O +V3+����س��ʱ�����ĵ缫��ӦʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����\(1)�����ײ��ϡ��ǵ�����Ͽ�ѧ�о���ǰ��,���о��ɹ��㷺Ӧ���ڴ��������¿�ѧ�С���ν�����ײ��ϡ���ָ�о�������������ֱ���Ӽ�������ʮ���IJ���,�罫���ײ��Ϸ�ɢ����ɢ����,���û������ܾ��е������� ____��
A.��ȫ������ֽ B.�ж����ЧӦ C.����Һ��ʽ�״ D.��������һ��������Һ
(2)�ѵ�����Һ���ڷ�ˮ��,�Ƴɵ��۽���,������Һ�͵��۽���������õķ�����__________��
(3)��������FeCl3������Һ�����ˮ��,�Ƴ�Fe(OH)3������������ϡ����ɹ۲쵽�������ǣ�__�������μ���ϡ��������ɹ۲쵽��������_____��д��������Ӧ�Ļ�ѧ����ʽ____��
��\����ͼ��~��ѡ���ʵ������ʣ�ʹ�����ߵ��������ܷ�����Ӧ����ѡ����Լ���ϡ���ᡢ������̼��ͭƬ��ʳ�Ρ���ʯ�ҡ�һ����̼�������Ƭ��ľ̿�ۡ�

��1�����ƶ����ǵĻ�ѧʽ�ֱ�Ϊ����______���� ______����_______��
��2��д���������֮��Ļ�ѧ����ʽ���ٺ͢ڣ�_______________���ں͢ܣ�____________���ۺ͢ܣ�______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ50.0mLNa2CO3��Na2SO4�Ļ����Һ���������BaCl2��Һ��õ�14.51g��ɫ�������� ����ϡ���ᴦ������������ٵ�4.66g����������ų����Լ��㣺
��1��ԭ�����Һ��Na2CO3��Na2SO4�����ʵ���Ũ��_______________��
��2�������������ڱ�״���µ����_____________��(Ҫ�й���)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com