
����Ŀ������ʪ���Ʊ������һ�ֹ����������£�

��֪��������Ҫ�ɷ�ΪCa5(PO4)3(OH)��������Ca5(PO4)3F���л�̼�ȡ��ܽ�ȣ�Ca5(PO4)3(OH)<CaSO4��0.5H2O
��1�������������ܼӿ췴Ӧ���ʵĴ�ʩ��___��
��2����������ʱ������Ӧ��2Ca5(PO4)3(OH)+3H2O+10H2SO4![]() 10CaSO4��0.5H2O+6H3PO4
10CaSO4��0.5H2O+6H3PO4
�ٸ÷�Ӧ���ֳ����Թ�ϵ��H3PO4___H2SO4���>����<������
�ڽ��Ԫ�������ɽ��͢��н��ۣ�P��S���Ӳ�����ͬ��___��
��3�����ʱ��������Ca5(PO4)3F������ת��ΪHF������һ��ת��ΪSiF4��ȥ��д������HF�Ļ�ѧ����ʽ��__��
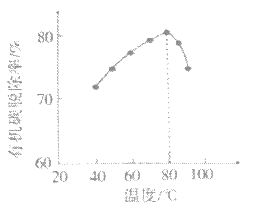
��4��H2O2���������е��л�̼����ΪCO2�ѳ���ͬʱ����Ҳ�ᷢ���ֽ⡣��ͬͶ�ϱȡ���ͬ��Ӧʱ�䣬��ͬ�¶��µ��л�̼�ѳ�����ͼ��ʾ��80����ѳ��ʱ仯��ԭ��___��
��5������ʱ��CaCO3�Թ�������ַ�Ӧ������SO42������������BaCO3�ɽ�һ���������ѳ��ʣ������ӷ���ʽ��__��
���𰸡���ĥ������ < �˵����P<S��ԭ�Ӱ뾶P>S���õ�������P<S���ǽ�����P<S 2Ca5(PO4)3F+10H2SO4+5H2O��10CaSO4��0.5H2O+6H3PO4+2HF�� 80���H2O2�ֽ����ʴ�Ũ���������� BaCO3+SO42��+2H3PO4��BaSO4+CO2��+H2O+2H2PO4��
��������
(1)���üӿ컯ѧ��Ӧ���ʵĴ�ʩ����ĥ�����ȣ��ܽ�ʱ����ȣ�
(2)�ٸ��ݷ�Ӧ����ʽ������H2SO4�μӷ�Ӧ�õ�H3PO4������ǿ����ȡ���
��H3PO4��H2SO4��Ϊ��ۺ����ᣬ��Ҫ�ɴ�P��S�ķǽ����ԽǶȿ��ǣ�S�ķǽ�����ǿ��P������ʹO�ϵ������ܶȽ����̶�����H+�����룬H+Խ���룬����������Խǿ��
(3)���ʱ��������Ca5(PO4)3F������ת��ΪHF����Ӧ����ΪCa5(PO4)3F��H2SO4��Ӧ������CaSO4![]() H2O��H3PO4��HF���ݴ�д����Ӧ����ʽ��
H2O��H3PO4��HF���ݴ�д����Ӧ����ʽ��
(4)����ͼ��80��ǰ�����¶����ߣ��л�̼�ѳ�������80��������¶����ߣ��л�̼�ѳ��ʽ��ͣ�����H2O2���ȷֽ⣬����H2O2Ũ�Ƚ���Ӱ���л�̼���ѳ��ʣ�
(5)��������Ǽ���CaCO3������Ӧ������ʱ��CaCO3�Թ��������������ᷴӦ����CaSO4����CaSO4�ܣ����Գ�ַ�Ӧ������SO42-����������BaCO3�ɽ�һ���������ѳ��ʣ�����BaCO3�μӷ�Ӧת���������ܵ�BaSO4���ڳ���ת���ķ�Ӧ����֮�Ϸ�����һ����Ӧ��ʹ������г̶����ݴ˷�����
(1)���üӿ컯ѧ��Ӧ���ʵĴ�ʩ����ĥ�����ȣ��ܽ�ʱ����ȡ��ʴ�Ϊ����ĥ�����ȣ�
(2)�ٸ��ݷ�Ӧ����ʽ������H2SO4�μӷ�Ӧ�õ�H3PO4������ǿ����ȡ���ᣬ�������ǿ��Ϊ��H3PO4��H2SO4��
��H3PO4��H2SO4��Ϊ��ۺ����ᣬ��Ҫ�ɴ�P��S�ķǽ����ԽǶȿ��ǣ�S�ķǽ�����ǿ��P������ʹO�ϵ������ܶȽ����̶�����H+�����룬H+Խ���룬����������Խǿ�����Լ���Ϊ��P�İ뾶����S��P�ķǽ�����С��S������H3PO4������С��H2SO4�����ԣ�
(3)���ʱ��������Ca5(PO4)3F������ת��ΪHF����Ӧ����ΪCa5(PO4)3F��H2SO4��Ӧ������CaSO4![]() H2O��H3PO4��HF�����Ի�ѧ��Ӧ����ʽΪ��2Ca5(PO4)3F+10H2SO4+5H2O
H2O��H3PO4��HF�����Ի�ѧ��Ӧ����ʽΪ��2Ca5(PO4)3F+10H2SO4+5H2O ![]() 10CaSO4
10CaSO4![]() H2O+6H3PO4+2HF��
H2O+6H3PO4+2HF��
(4)����ͼ��80��ǰ�����¶����ߣ��л�̼�ѳ�������80��������¶����ߣ��л�̼�ѳ��ʽ��ͣ�����H2O2���ȷֽ⣬����H2O2Ũ�Ƚ���Ӱ���л�̼���ѳ��ʣ����Կ��Խ���Ϊ���¶ȸ���80��ʱ��H2O2�ķֽ����ʼӿ죬����H2O2��Ũ�Ƚ��ͣ�Ҳ�͵����л�̼�ѳ����½���
(5)����BaCO3�ɽ�һ���������ѳ��ʣ�����BaCO3�μӷ�Ӧת���������ܵ�BaSO4��BaSO4�����CaSO4�ܽ�ȸ��ͣ���ʹ��Һ��SO42-Ũ�ȸ��ͣ����ǵ�����ת���ķ�ӦЧ�ʽϵͣ����Խ�һ���������ѳ������ǿ����ڳ���ת����Ӧ�Ļ����Ϸ���Ч�ʸ��ߵķ�Ӧ����ʹ������Ӧ�Ľ��У�����ת����ӦΪ��BaCO3+SO42-![]() BaSO4+CO32-����ʱ��Һ���������һ�������ԣ����Լ���������Ӧ����CO2������������Ӧ��������������еij̶ȸ����Դﵽ��һ���ѳ��ʵ�Ч�����ۺϿ��������ӷ���ʽΪ��BaCO3+SO42-+H3PO4�TBSO4+CO2��+H2O+H2PO4-��
BaSO4+CO32-����ʱ��Һ���������һ�������ԣ����Լ���������Ӧ����CO2������������Ӧ��������������еij̶ȸ����Դﵽ��һ���ѳ��ʵ�Ч�����ۺϿ��������ӷ���ʽΪ��BaCO3+SO42-+H3PO4�TBSO4+CO2��+H2O+H2PO4-��


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ҫ�ɷ�ΪFe2O3��Fe3O4���Լ�����SiO2��Al2O3�ȡ��������������Ʊ����죨Fe2O3����һ�ֹ����������£�

��֪����ԭ����ʱ����Fe2O3��Fe3O4ת��ΪFeO��
�������ӿ�ʼ��������ȫ����ʱ��pH���±���ʾ��
���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
Fe2+ | 7.6 | 9.7 |
Fe3+ | 2.7 | 3.7 |
Al3+ | 3.8 | 4.7 |
��1���������������������������Һ�еĽ����������У������ӷ��ţ�________________��
��2��Fe�۳���pH�⣬��һ��������___________��Fe�۵�����Һ��pHΪ__________��
��3��������������������������FeCO3�����ӷ���ʽΪ_______________________________��
������Һ����Ҫ�����ǣ��ѧʽ��_______________��
��4�����������£��������������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��BaTiO3��KH2PO4��NaNO2�����������壬���Ǿ�����������������統����ѹ�����ı���״ʱ����������ͨ��ʱ��ı���״�ȡ�
(1)��̬Tiԭ�ӵļ۵����Ų�ʽΪ___________������___________��Ԫ�ء�
(2)KH2PO4�����д��ڵ���������___________(ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.���
(3) NaNO2��Nԭ�ӵ��ӻ�����Ϊ___������(�>��<��=��)____120�㣬��ԭ����___________��N��O��Na����Ԫ�صĵڶ�������(I2)�ɴ�С��˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(4)BaTiO3�ľ�������ͼ��ʾ��

Tiԭ�ӵ���λ��Ϊ_____��������ܶ�Ϊ��g/cm3�������Baԭ�Ӻ�Oԭ��֮��ľ���Ϊ___�������ʽ)nm��( BaTiO3��Ħ������Ϊ233g/mol��NAΪ�����ӵ�������ֵ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(NH4)2SO3�����ǰ����������Ҫ���̡�ijС����������������ʱ���ֱ��о���һ��ʱ�����¶Ⱥ�(NH4)2SO3��ʼŨ�ȶԿ�������(NH4)2SO3���ʵ�Ӱ�죬�����ͼ������˵������ȷ����
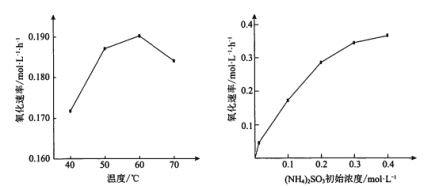
A.60��֮ǰ�����������������¶�����ѧ��Ӧ���ʼӿ��й�
B.60��֮���������ʽ��Ϳ�����O2���ܽ���½���(NH4)2SO3�����ֽ��й�
C.(NH4)2SO3��ʼŨ������һ���̶ȣ��������ʱ仯������SO32-ˮ��̶������й�
D.(NH4)2SO3��ʼŨ������һ���̶ȣ��������ʱ仯��������O2���ܽ������й�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.2019����Ԫ�����ڱ�����150���꣬�ڼ��ѧ��Ϊ�������ڱ������˲�иŬ�����й���ѧԺԺʿ���������������ֲⶨ������49In����9��Ԫ�����ԭ����������ֵ��������Ϊ�����±���
��1��In�����ڱ��е�λ����___��
��2��In������������ˮ����ļ���___Ba(OH)2�ļ��ԣ��>����<������
��3��![]() In����������������IJ�ֵΪ___��
In����������������IJ�ֵΪ___��
II.A+��B2����C����D��E��F3+�ֱ��ʾ��10�����ӵ��������ӣ����ӻ���ӣ������У�
a.A+��B2����F3+������Ӳ�ṹ��ͬ
b.C����������Ԫ����ɵ�
C.D������Ԫ����ɵ���ԭ�ӷ���
d.E�ڳ���������ɫҺ��
e.����F3+����Һ�еμӺ�C������Һ�����������а�ɫ�������ɣ����ɫ������ʧ
��4��C���ĵ���ʽ��___��
��5��A+��B2����F3+���Ӱ뾶�ɴ�С��˳��Ϊ___�������ӷ��ű�ʾ����
��6������ʽ��ʾA2B���γɹ���___��
��7����F3+����Һ��ͨ�����D����Ӧ�����ӷ���ʽ��___��
��8��A������E��Ӧ�����ӷ���ʽΪ___�����ɵĻ������л�ѧ����������___��
III.����34Se���Ƕ�����������������Ԫ��֮һ��Ҳ����Ҫ�Ĺ�ҵԭ�ϣ�����ͬ�塣
��9��Seԭ�ӽṹʾ��ͼ�ɱ�ʾΪ___��
��10������˵����������___��
a.SeO2�������������л�ԭ��
b.Ũ������ܾ���ǿ�����ԡ���ˮ��
c.���ȶ��ԣ�H2Se<HCl<H2S
d.���ԣ�H2SeO4<HBrO4<HClO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
���Ʊ�Na2S2O35H2O��Ӧԭ����Na2SO3(aq)��S(s)![]() Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
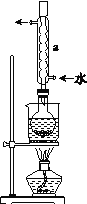
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O35H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����a��������_________����������__________________________��
(2)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������________�������Ƿ���ڸ����ʵķ�����________________________________��
(3)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________��
�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 molL-1��ı���Һ�ζ�����Ӧԭ��Ϊ2S2O32-��I2��S4O62-��2I-��
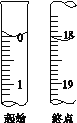
(4)�ζ����յ�ʱ���ж�������___________________________��
(5)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ_______mL����Ʒ�Ĵ���Ϊ(��Na2S2O35H2O��Է�������ΪM)_________��(�ú�M��ʽ�ӱ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ܡ��������ʷdz����ƣ����ǵĻ�����Ӧ��ʮ�ֹ㷺���ش���������:
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ_______�������ܡ����Ļ�̬ԭ�Ӻ���δ�ɶԵ�����������________��
��2��CoCl2���ڰ�ˮ��ͨ��������ɴ���Һ�нᾧ���Ȼ�ɫ��[Co(NH3)6]Cl3���塣���������������ӵ����幹����_______��������ԭ�ӵ��ӻ��������Ϊ_________��
��3������������CO�����γ��ʻ������Fe(CO)5��Ni(CO)4,Fe(CO)5���۵�Ϊ253K, �е�Ϊ376K,��Ni(CO)4��������_____���壬���д��ڵĻ�ѧ������Ϊ_________��
��4��NiO��FeO�ľ���ṹ�������Ȼ��Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm,���۵�NiO_______FeO (�� ��>����<�� ����=��)��ԭ����___________��
��5��Fe3O4�����У�O2-���ظ����з�ʽ��ͼ��ʾ�������з�ʽ�д���������1��3��6��7��O2-Χ�ɵ����������϶��3��6��7��8��9��12��O2-Χ�ɵ����������϶��Fe3O4����һ���Fe3+��������������϶�У���һ��Fe3+��Fe2+��������������϶�У���Fe3O4�����У����������϶����O2-��֮��Ϊ______����_____%�����������϶û����������ӡ�Fe3O4��������8��ͼʾ�ṹ��Ԫ�������ܶ�Ϊ5.18g/cm3,��þ�������a=_____pm��(д���������ʽ)
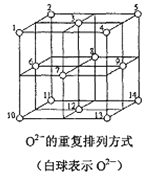
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�أ���֪��![]() Ԫ��ԭ�Ӽ۵����Ų�ʽΪ
Ԫ��ԭ�Ӽ۵����Ų�ʽΪ![]() ����ԭ�Ӱ뾶��ͬ��Ԫ������С�ġ�
����ԭ�Ӱ뾶��ͬ��Ԫ������С�ġ�![]() Ԫ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺����С��YԪ�أ���Wԭ�ӵĵ����Ų��У�p�����ֻ��һ��δ�ɶԵ��ӡ�
Ԫ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺����С��YԪ�أ���Wԭ�ӵĵ����Ų��У�p�����ֻ��һ��δ�ɶԵ��ӡ�![]() Ԫ�صĵ������������
Ԫ�صĵ������������![]() ��
��
|
|
|
|
|
496 | 4562 | 6912 | 9540 |
|
��ش�
![]() �ĵ���ʽΪ______�����еĻ�ѧ������Ϊ______��
�ĵ���ʽΪ______�����еĻ�ѧ������Ϊ______��![]() Ϊ______���塣
Ϊ______���塣
![]() ��ˮ����ǿ��ˮ�����һ������A����Һ�ʼ��ԣ���A�ĽṹʽΪ______������ӿռ乹��Ϊ______��
��ˮ����ǿ��ˮ�����һ������A����Һ�ʼ��ԣ���A�ĽṹʽΪ______������ӿռ乹��Ϊ______��
![]() ��Y��Z��W����Ԫ�����γɵĵ����У�Ӳ��������______
��Y��Z��W����Ԫ�����γɵĵ����У�Ӳ��������______![]() �����ʵ�����
�����ʵ�����![]() ������
������![]() ���Ըߵ�ԭ����______��
���Ըߵ�ԭ����______��![]() ��Xԭ�ӵ��ӻ��������Ϊ______��
��Xԭ�ӵ��ӻ��������Ϊ______��
![]() ����Ľṹʾ��ͼ��ͼ��ʾ����þ����Ħ������Ϊ
����Ľṹʾ��ͼ��ͼ��ʾ����þ����Ħ������Ϊ![]() ��������ܶ�Ϊ
��������ܶ�Ϊ![]() �����ӵ�����Ϊ
�����ӵ�����Ϊ![]() �����������������Z�������ļ�ľ���
�����������������Z�������ļ�ľ���![]() ______��
______��
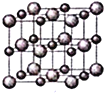
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ѧ������Ҫ���ã�Ҳ����Ҫ�Ļ���ԭ�ϣ���˳�Ϊ���������о��ȵ㡣��ͼ�ǻ����������ַ���������A��һ�ֳ������������IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��
(1)�����л�������һ�ֿ���Ϊˮ���Ĵ��������ṹ��ʽ��___________��������ȫˮ��õ�D��D��������__________�������Ƿ��е��۵��Լ���_________________________��
(2)A��B�ķ�Ӧ������_______��B��C��Ӧ�Ļ�ѧ����ʽ��__________________________________��
(3)B���Ա����Ը��������Һ�������ظ������Һֱ������Ϊ�л���E��B��E����Ũ����������·�Ӧ������(C4H8O2)����֪ʵ����60gE������B��Ӧ������66 g������������IJ���(ʵ�ʲ���ռ���۲����İٷ���)Ϊ______________��
(4)Ϊ�������ˮ����С���������Һ�м���ϡ���Ტ����һ��ʱ�䣬��ȴ�����Һ��ֱ�Ӽ�������������ͭ����Һ�����ȣ���ש��ɫ�������֣�����ΪС��ʵ��ʧ�ܵ���Ҫԭ����_______
(5)����������Ӧ���Ʊ�һ�ֲ���ҩ�����ʽΪ(δ��ƽ)��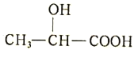 +Fe��
+Fe��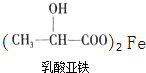 +X����45g����������Ӧ���� X �����Ϊ_______L(��״��)��
+X����45g����������Ӧ���� X �����Ϊ_______L(��״��)��
(6)������������һ��������ͨ��������Ӧ������һ������Ԫ����(C6H8O4)��������ˮ���û����Ľṹ��ʽ��________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com