
����Ŀ����Ҫ��ش��������⡣
(1)Al2(SO4)3��Һ�����Ե����ӷ���ʽ��____________________________��
(2)CuSO4��Һ�������ˮ��Ӧ�����ӷ���ʽ��____________________________��
(3)Mg-Al-NaOH��Һ��ɵ�ԭ��أ������ĵ缫��Ӧʽ��_________________________��
(4)CH3OH-O2ȼ�ϵ�أ�KOH��Һ������ʣ������ĵ缫��Ӧʽ��____________________��
(5)���Ե缫���CuSO4��Һ���ܷ�Ӧ�Ļ�ѧ����ʽ��______________________________��
(6)Na2C2O4��Һ�������غ㣺______________________________��
(7)Fe3+�Ļ�̬�����Ų�ʽ��______________________________��
(8)N2H4�Ľṹʽ��______________________________��
���𰸡�Al3++3H2OAl(OH)3+3H+ Cu2++4NH3H2O=[Cu(NH3)4]2++4H2O Al-3e-+4OH-�TAlO2-+2H2O CH3OH+8OH--6e-=CO32-+6H2O 2CuSO4+2H2O![]() 2Cu+2H2SO4+O2�� c(Na+)=2[c(C2O42-)+c(HC2O4-)+c(H2C2O4)] [Ar]3d5
2Cu+2H2SO4+O2�� c(Na+)=2[c(C2O42-)+c(HC2O4-)+c(H2C2O4)] [Ar]3d5 ![]()
��������
(1)Al2(SO4)3��Һ�д��������ӵ�ˮ�⣬������Һ�����ԣ��ʴ�Ϊ��Al3++3H2OAl(OH)3+3H+��
(2)CuSO4��Һ�������ˮ��Ӧ������ͭ�������ӣ��ʴ�Ϊ��Cu2++4NH3H2O=[Cu(NH3)4]2++4H2O��
(3)Mg-Al-NaOH��Һ��ɵ�ԭ��أ��ܷ�ӦΪAl������������Һ��Ӧ����ƫ�����ƺ������ķ�Ӧ��Alʧ���ӱ��������������ʴ�Ϊ��Al-3e-+4OH-�TAlO2-+2H2O��
(4)CH3OH-O2ȼ�ϵ�أ�KOH��Һ������ʣ������״�ʧȥ�������ɶ�����̼�����ڵ����Ϊ����������Һ�����Է�Ӧ������̼������ӣ������ĸ����缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
(5)�������ͭ��Һ����ͭ���ʡ����������ᣬ�ʴ�Ϊ��2CuSO4+2H2O![]() 2Cu+2H2SO4+O2����
2Cu+2H2SO4+O2����
(6)Na2C2O4��Һ�������ӵ�Ũ�ȵ��ں�̼ԭ������Ũ��֮�͵Ķ������ʴ�Ϊ��c(Na+)=2[c(C2O42-)+c(HC2O4-)+c(H2C2O4)]����
(7)FeԪ��Ϊ26��Ԫ�أ�ʧȥ�����������������Fe3+���ʻ�̬�����Ų�ʽΪ��[Ar]3d5��
(8) N2H4�ǹ��ۻ������ԭ�Ӻ���ԭ���γɹ��ۼ�����ԭ�Ӻ͵�ԭ�Ӽ�Ҳ�γɹ��ۼ����ṹʽΪ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3��5H2O)�׳�����������Ӧ�÷dz��㷺����ҵ�Ͽ������������Ʒ�(�������ƺ����ͨ�����Ϸ�Ӧ)�Ƶã�װ����ͼ(a)��ʾ��
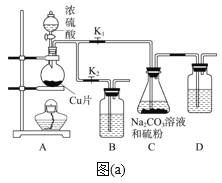
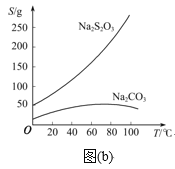
��֪��Na2S2O3��������Һ�в����ȶ����ڣ��й����ʵ��ܽ��������ͼ(b)��ʾ��
��1��Na2S2O3��5H2O���Ʊ���
����1����ͼ���Ӻ�װ�ú��A��Cװ�������ԵIJ�����_____��
����2������ҩƷ����K1���ر�K2����Բ����ƿ�м�������Ũ���Ტ���ȡ�װ��B��D��������________��
����3��C�л��Һ��������������Ӧһ��ʱ�����۵������١���C����Һ��pH�ӽ�7ʱ����K2���ر�K1��ֹͣ���ȣ�C����ҺҪ����pH��������_____��
����4������C�еĻ��Һ������Һ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��2��Na2S2O3���ʵļ��飺
��������������ˮ�еμ�Na2S2O3��Һ����ˮ��ɫ��dz��������Һ�еμ���������Һ���۲쵽�а�ɫ�����������ݴ���ΪNa2S2O3���л�ԭ�ԡ��÷����Ƿ���ȷ��˵�����ɣ�____��
��3������Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȣ��������£�ȡ��ˮ25.00mL�������ʵ�����ȼ�������K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټӹ���KI��Һ����ַ�Ӧ���������Һ��ָʾ������0.010mol��L-1��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ������Na2S2O3��Һ18.00mL�����ַ�Ӧ�����ӷ���ʽΪ��a.Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O��b.I2+2S2O32-=2I-+S4O62-����÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ŷ�����п��һ�ֲ�п����������![]() �����Ŷ�����Ϊԭ���Ʊ���
�����Ŷ�����Ϊԭ���Ʊ���
(1)![]() ��̬��������Ų�ʽΪ_______��
��̬��������Ų�ʽΪ_______��
(2)![]() �Ŀռ乹��Ϊ________(����������)����
�Ŀռ乹��Ϊ________(����������)����![]() ��Ϊ�ȵ������һ�������ӵĻ�ѧʽΪ________��
��Ϊ�ȵ������һ�������ӵĻ�ѧʽΪ________��
(3)���Ŷ�����( )�����е�ԭ�ӵĹ���ӻ�����Ϊ________�����Ŷ�����_______(��������������������)��
)�����е�ԭ�ӵĹ���ӻ�����Ϊ________�����Ŷ�����_______(��������������������)��![]() ���ᡣ
���ᡣ
(4)ZnO�ľ����ṹ��ͼ��ʾ��![]() ����λ��Ϊ________��
����λ��Ϊ________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�Ka(HCOOH)=1.77��10-4��Ka(CH3COOH)=1.75��10-5������˵������ȷ����
A.Ũ�Ⱦ�Ϊ0.1mol��L-1��HCOONa��CH3COONa��Һ�����ӵ����ʵ���Ũ��֮�ͣ�ǰ�ߴ��ں���
B.����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ������pH��Ϊ3��HCOOH��CH3COOH��Һ���յ㣬����NaOH��Һ�������ǰ��С�ں���
C.0.2mol��L-1 HCOOH��0.1mol��L-1 NaOH�������Ϻ����Һ�У�c(HCOO-)+c(OH-)=c(HCOOH)+c(H+)
D.0.2mol��L-1 CH3COONa �� 0.1mol��L-1����������Ϻ����Һ�У�pH<7����c(CH3COO-)>c(Cl- )>c(CH3COOH)>c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�������Ա�о�����Cu-ZnO-ZrO2������CO2�����Ƽ״�������ˮ�����û�����������Ӧ������ͼ��ʾ��H2��![]() H+
H+![]() H��������˵��������ǣ� ��
H��������˵��������ǣ� ��

A.������̼�����Ƽ״��Ĺ�����ԭ�������ʴ�100%
B.����ǵ������Ǹ÷�Ӧ�����е��м����
C.��÷�Ӧ��ϵ�м���������ˮ�����Ӽ״�������
D.�ڢ۲��ķ�ӦʽΪ![]() H3CO+H2O��CH3OH+
H3CO+H2O��CH3OH+![]() HO
HO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A. ������FeCl3��Һ�����ˮ���Ʊ�������Fe3++3H2O![]() Fe(OH)3��+3H+
Fe(OH)3��+3H+
B. ��FeSȥ����ˮ�е�Hg2+��Hg2+ + S2- === HgS��
C. AlCl3��Һ��Na2S��Һ������ɳ���:��2 Al3++3S2-==Al2S3��
D. NH4Cl����D2O����������NH4++D2O![]() NH3��HDO+D+
NH3��HDO+D+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ��CuS��MnS��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵���������
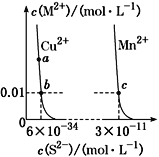
A. �ں���CuS��MnS�������Һ��c(Cu2��)��c(Mn2��)��1��2��10��23
B. ��CuSO4��Һ�м���MnS������Ӧ��
Cu2��(aq)��MnS(s)![]() CuS(s)��Mn2��(aq)
CuS(s)��Mn2��(aq)
C. a���Ӧ��Ksp����b���Ӧ��Ksp
D. ���¶��£�Ksp(CuS)С��Ksp(MnS)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʽ̼��ͭ�������л�������ɱ�����������ͭ�����Ӽ������������̻���������졣CuSO4��Һ��Na2CO3��Һ��Ӧ�ܷ�õ���ʽ̼��ͭ��ij��ͬѧ�������̽����
�������Ʊ���ȡһ����������CuSO4��5H2O������ˮ���ٵμ���ϡ���ᣬ��ֽ����õ�һ�������CuSO4��Һ�������м�����Na2CO3��Һ������������ɫ����Һ���ˣ�������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӡ�
��1���μ�ϡ�����������________��
��2������ˮ�Ҵ�ϴ�ӵ�Ŀ����________��
��ʵ��̽����ͬѧ�����������װ�ã����Ƶõ�����ɫ�������ʵ�飺
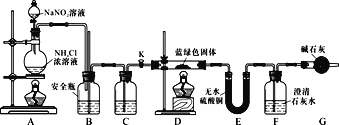
��3��Dװ�ü���ǰ����Ҫ���ȴ���K����Aװ����ȡ����N2��Ȼ��ر�K����ȼD���ƾ��ơ�A�в�����N2��װ���еĿ����ų���������š�C��ʢװ���Լ�Ӧ��________��
��4��װ��A�з�����Ӧ�����ӷ���ʽΪ________��
��5��������ɫ��������ΪxCuCO3��yCu(OH)2��ʵ���ܹ۲쵽��������________��
��6��ͬѧ�Dz�������֪��Ksp(CaCO3)��2.8��10��9��Ksp(BaCO3)��5.1��10-9����������Ϊ��Ҫ��Ba(OH)2����Ca(OH)2�������ⶨ����ɫ����Ļ�ѧʽ����ԭ����________��
a��Ba(OH)2�ļ��Ա�Ca(OH)2ǿ
b��Ba(OH)2�ܽ�ȴ���Ca(OH)2���ܳ������CO2
c����ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
d�����յ���CO2���ɵ�BaCO3����������CaCO3���������С
��D�з�Ӧ��ȫ����K���ٴεμ�NaNO2��Һ����N2����Ŀ����________��������������ȡ����ɫ��������Ϊ27.1 g��װ��F��ʹ��Ba(OH)2��Һ��ʵ�������װ��E����������2.7 g��F�в�������19.7 g���������ɫ����Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Mn(H2PO4)2��2H2O��һ�ְ�ɫ���壬������ˮ�������ڻ�е�豸���������������̿�(��Ҫ�ɷ�ΪMnO2��������������Fe2O3��FeO��Al2O3)Ϊԭ���Ʊ�Mn(H2PO4)2��2H2O��������ͼ��
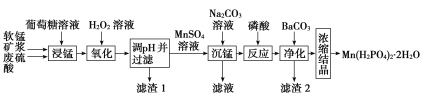
��1�����̿�Ҫ���Ƴɿ�Ŀ����__��������(C6H12O6)��MnO2��Ӧʱ������ΪMnSO4��CO2��H2O���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__��
��2����H2O2��Һ��������ʱ������Ӧ�����ӷ���ʽΪ��__��
��3����֪���ֽ������ӵ��������↑ʼ��������ȫ������pH���������pH��������ʱ��Ӧ������pH��ΧΪ__������1����Ҫ�ɷ�Ϊ__(�ѧʽ)��
�������� | ��ʼ������pH | ��ȫ������pH |
Fe3+ | 1.8 | 3.2 |
Al3+ | 3.0 | 5.0 |
Fe2+ | 5.8 | 8.8 |
Mn2+ | 7.8 | 9.8 |
��4���������������Ӧ�Ļ�ѧ����ʽΪ__��
��5��ij���������������Ʊ�Mn(H2PO4)2��2H2O����֪���̿���MnO2�ĺ���Ϊ87%��������������Ԫ�ص������Ϊ9%����1�ָ����̿���Ƶ�Mn(H2PO4)2��2H2O__t��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com