
����Ŀ��H2C2O4 (��������Ԫ����)���ڱ���������ؾ�����Ϊ�궨NaOH��ҺŨ�ȵĻ����ʣ��Ӷ����NaOH����Һ��
��1����ˮ��Һ��H2C2O4�ĵ��뷽��ʽΪ________��
��2����0.1molL-1NaOH��Һ�ζ�0.1molL-1������Һ�ĵζ���������ͼ��ʾ��

�ٵζ������д�X�㵽Y�㣬��Ӧ�����ӷ���ʽΪ________��
����NaOH��Һ�ζ�������������������ƿ����Һ��ˮ�ĵ���̶� _______(�����)��
a.ʼ�ռ�С b.ʼ������ c.�ȼ�С������ d.��������С
��X��ʱ��c(Na+)-c(C2O42-)_____c(H2C2O4) +c(HC2O4-) (ѡ������������=);Y��ʱ��c(OH-) - c(H+) _______c(H2C2O4)+ c(HC2O4-) (ѡ������������=)��
��3��ijͬѧ���ڱ����������(![]() ��Ħ������Ϊ204gmol-1��������ˮ�Ĺ��壬ˮ��Һ��������)�궨NaOH��Һ����Ҫʵ�鲽��������
��Ħ������Ϊ204gmol-1��������ˮ�Ĺ��壬ˮ��Һ��������)�궨NaOH��Һ����Ҫʵ�鲽��������
������.ȷ��ȡ0.4896g�ڱ��������������ƿ�У���������ˮ�ܽ�
��.����1~2�η�̪��ָʾ��
��.�ô���NaOH��Һ�ζ�����
���жϴﵽ�ζ��յ�ķ�����___________��
�����ζ����յ�����NaOH��ҺΪ25.00mL����ôεζ���õ�NaOH��ҺŨ��Ϊ _______��
����������ᵼ�²�õ�NaOH��ҺŨ��ƫ�����__________(�����)��
a.�ζ���δ�ô���NaOH��Һ��ϴ
b.�ζ�ǰ�ζ��ܼ�˲��������ݣ��ζ���������ʧ
c.�ζ���������������ˮϡ����ƿ����Һ
d.����ʱ���ζ�ǰ���ӵζ��̶ܿȣ��ζ���ƽ�ӵζ��̶ܿ�
���𰸡� H2C2O4![]() H++HC2O4-��HC2O4-
H++HC2O4-��HC2O4- ![]() H++C2O42- HC2O4-+OH-=C2O42-+H2O d = �� �μ����һ��NaOH��Һʱ����ƿ����Һ����ɫ���Ұ�����ڲ���ɫ 0.09600molL-1 d
H++C2O42- HC2O4-+OH-=C2O42-+H2O d = �� �μ����һ��NaOH��Һʱ����ƿ����Һ����ɫ���Ұ�����ڲ���ɫ 0.09600molL-1 d
��������(1)�����Ƕ�Ԫ���ᣬ��ˮ��Һ������������룬��һ������̶ȴ��ڵڶ���������뷽��ʽ�ֱ�Ϊ��H2C2O4![]() HC2O4-+H+��HC2O4-
HC2O4-+H+��HC2O4-![]() C2O42-+H+���ʴ�Ϊ��H2C2O4
C2O42-+H+���ʴ�Ϊ��H2C2O4![]() HC2O4-+H+��HC2O4-
HC2O4-+H+��HC2O4-![]() C2O42-+H+��
C2O42-+H+��
(2)�ٵζ������д�X�㵽Y�㣬Ϊ�������ƺ��������Ʒ�Ӧ�����ӷ�ӦΪ��HC2O4-+OH-=C2O42-+H2O���ʴ�Ϊ��HC2O4-+OH-=C2O42-+H2O��
����NaOH��Һ�ζ�����ʼ��Һ�����ԣ��ζ�����������������Ũ�ȼ�С��ˮ�ĵ���̶������ζ���ȫ��NaOH��Һ�ζ�����������Һ�ʼ��ԣ�����ˮ�ĵ��룬��������������ƿ����Һ��ˮ�ĵ���̶�ʹ��������С���ʴ�Ϊ��d��
��X��ʱ��ҺΪNaHC2O4��Һ��HC2O4-����Һ�з���������ˮ�⣬����ƽ�⣺HC2O4-+H2OH2C2O4+OH-��̼Ԫ������Һ�д�����ʽ�У�HC2O4-��H2C2O4��C2O42-�����������غ���c(Na+)=c(HC2O4-)+c(H2C2O4)+c(C2O42-)����c(Na+)-c(C2O42-)=c(H2C2O4)+c(HC2O4-)��Y��ʱΪNa2C2O4��Һ�����ݵ���غ��У�c(Na+)+c(H+)=c(HC2O4-)+2c(C2O42-)+c(OH-)�٣������غ���c(Na+)=2[c(HC2O4-)+c(H2C2O4)+c(C2O42-)]�ڣ����ڴ���ٵ�c(OH-)-c(H+)=c(H2C2O4)+2c(HC2O4-)������c(OH-)-c(H+)��c(H2C2O4)+c(HC2O4-)���ʴ�Ϊ��=������
(3)���ڱ����������Ϊ���ᣬ��̪��pH��8ʱΪ��ɫ��pHΪ8��10֮�䣬��dz��ɫ�������ô���NaOH��Һ�ζ����յ㣬����ɫ��Һ�����ɫ���Ұ�����ڲ���ɫ��˵����Ӧ���յ㣬�ʴ�Ϊ���μ����һ��NaOH��Һʱ����ƿ����Һ����ɫ���Ұ�����ڲ���ɫ��
��0.4896g![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() =0.0024mol��
=0.0024mol��
![]() +NaOH��
+NaOH��![]() +H2O
+H2O
1 1
0.0024mol 0.025L��c(NaOH)
��0.0024mol =0.025L��c(NaOH)����ã�c(NaOH)=0.09600 mol/L�ʴ�Ϊ��0.09600molL-1��
��a����ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ����������NaOH��ҺŨ�Ƚ��ͣ���a����b���ζ�ǰ�ζ��ܼ�˲��������ݣ��ζ��յ�ʱ������ʧ�����V(�����)ƫ����c(�����)= 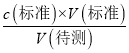 ������֪c(�����)ƫС����b����c���ζ���������������ˮϡ����ƿ����Һ����Һ�����ʵ������䣬����c(�����)=
������֪c(�����)ƫС����b����c���ζ���������������ˮϡ����ƿ����Һ����Һ�����ʵ������䣬����c(�����)= 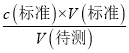 ������֪��c(����)���䣬��c����d������ʱ���ζ�ǰ���ӵζ��̶ܿȣ��ζ���ƽ�ӵζ��̶ܿȣ����V(�����)��Һ���ƫС������c(�����)=
������֪��c(����)���䣬��c����d������ʱ���ζ�ǰ���ӵζ��̶ܿȣ��ζ���ƽ�ӵζ��̶ܿȣ����V(�����)��Һ���ƫС������c(�����)= 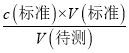 ������֪c(�����)ƫ��d��ȷ���ʴ�Ϊ��d��
������֪c(�����)ƫ��d��ȷ���ʴ�Ϊ��d��


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ױ������ϵ���ԭ�ӱ�������ԭ��ȡ�������ɵĶ��������6�֣���ױ������ϵ���������У� ��
A.3��
B.4��
C.5��
D.6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������İ�������һ����Ҫ�����Ǽ�������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ�������йظ����ʵ�˵����ȷ���ǣ� ��
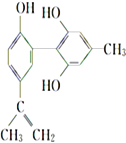
A. ��FeCl3��Һ����ɫ����Ϊ�������뱽������ͬϵ��
B. �÷����е�����̼ԭ��һ����ƽ��
C. 1 mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ���������Br2��H2�ֱ�Ϊ4 mol��7 mol
D. ����KMnO4������Һ���۲쵽��ɫ��ȥ����֤���ṹ�д���̼̼˫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йء��͡���˵����ȷ���ǣ� ��
A.��֬���������͡�ֲ�����붯����
B.��֬���⻯����֬�����������ڼӳɷ�Ӧ
C.ֲ������ʹ�����ɫ
D.��֬���ڸ߷��ӻ�������Է���ˮ�ⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵����ȷ����
A. �����£�pH��Ϊ9��CH3COONa��NaOH��Һ�У�ˮ�ĵ���̶Ȳ���ͬ
B. ��ӦNH3(g)+HCl(g)��NH4Cl(s)�������¿��Է����У���÷�Ӧ����H��0
C. ��Ũ�Ⱦ�Ϊ0.1 molL-1��MgCl2��CuCl2�����Һ����μ��백ˮ���ȳ�����ɫ������˵��Ksp[Mg(OH)2]>Ksp[Cu(OH)2]
D. ��ʢ��KI3��Һ(I3-![]() I2��I-)���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2��I-)���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����18.4g����ͭ��ɵĺϽ��м��������������Һ���Ͻ���ȫ�ܽ⣬ͬʱ����NO2��NO������壬����������Һ�м���������NaOH��Һ������30.3g��������ȡ�������ĺϽ�ʹ����һ����������ǡ����ȫ��Ӧ��������������ڱ�״����Ϊ�� ��
A.7.84L
B.6.72L
C.4.48L
D.������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2CO3��MaHCO3��xNa2CO3��yH2O(��̼���)�ڹ�ũҵ��������;�dz��㷺��
��1��0.1mol/LNa2CO3��Һ��ˮϡ��ʱ����Һ��pH____ (����������������С������������)��
��2��25��ʱ��H2CO3�ĵ��볣��Ka1=5��10-7��Ka=5��10-11��NH3��H2O�ĵ��볣��Kb=1.8��10-5����������ƽ�ⳣ����
��NaHCO3ˮ�ⷴӦHCO3-+H2O![]() H2CO3+OH-��ƽ�ⳣ��ΪK= ________ ��������ֵ��
H2CO3+OH-��ƽ�ⳣ��ΪK= ________ ��������ֵ��
�ڷ�ӦHCO3-+NH3��H2O![]() CO32-+NH4++H2O��ƽ�ⳣ��ΪK=_________�� ������ֵ��
CO32-+NH4++H2O��ƽ�ⳣ��ΪK=_________�� ������ֵ��
��3��һ�ֲⶨxNa2CO3��yH2O2��y/xֵ�ķ���������

�ٵζ�CO32-ʱ���յ���Һ��ɫ�仯��______��
�ڵζ�H2O2ʱ��MnO4-����ԭΪMn2+����Ӧ�����ӷ���ʽΪ_______��
������������25.00mL������KMnO4��Һ19.00mL��y/x��ֵ(�г��������)__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A��Ħ���DZ�ʾ���ʵ�������λ
B��1 mol���������2 g
C��O2��Ħ��������32 g
D��ÿĦ�����ʶ����а����ӵ�������ָ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ�����ڱ��ļ��Կ��ͼ��
��1����������Ԫ�����ڱ��л�������Ԫ����ǽ���Ԫ�صķֽ��� �� �������Ų����ɰ�Ԫ�ػ��ֳ�5��������ȫ�ǽ���Ԫ�ص���Ϊ ��
��2���ϱ���Ԫ�آ١���ԭ�ӵ��������ӵĵ����Ų�ʽ�ֱ�Ϊ�����Ƚ�Ԫ�آ���Ԫ�آڵ��������ʣ���д��������������ԭ�Ӱ뾶�����ڡ��縺�ԣ����ڡ������ԣ����ڣ�
��3��ij������Ԫ���������Ϊ+7����ԭ�ӽṹʾ��ͼΪ ��
��4��������Ԫ���������������۵ľ���ֵ��ȣ�����Ϊ�ɰ���Ԫ�ط������ڱ��е��壻���˽��齫��Ԫ������Ԫ�����ڱ��Ģ�A�壬����д��֧����һ�۵��1����ѧ��ʵ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com