
����Ŀ��ʵ��������500mL 0.2mol/L��NaOH��Һ��
(1)����ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_____________(�����)����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��____________��
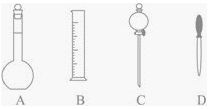
(2)��д���������еĿհף�
���岽�����£�
�ټ�����Ҫ����NaOH���������___________g��
����������ƽ����NaOH���壻
�۽��ƺõ�NaOH��������ձ��У�����������ˮ�ܽ⡢���裬��_________�����£�
�ܽ�NaOH��Һ�ز�����ע��____________�У�
������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲ��ע������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�
������ˮע������ƿ��Һ����̶�����_______cmʱ������____________�μ�����ˮ��Һ���ڿ̶������У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ�
(3)����ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L��ԭ�������_____________
A.ʹ����ֽ����NaOH���壻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ�
C.����ƿ��ԭ������������ˮ��
D.����ʱ���õ��������⣻
E.δ��ȴֱ��ת��������ƿ��������á�
���𰸡�C �ձ� ������ 4.0 ��ȴ 500mL����ƿ 1��2 ��ͷ�ι� AB
��������
(1)����ʵ������IJ��裨���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������Լ�ÿ��������Ҫ����ȷ����Ӧ����������
(2)����m=c��V��M������Ҫ���ʵ���������������һ�����ʵ���Ũ����Һ��һ�㲽��ȷ��ʹ�õ�������������Ҫ��
(3)�������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(1)����һ�����һ�����ʵ���Ũ�ȵ���ҺҪ��һ�����������ƿ�н��У�������ƽ�������ʣ�����Ͳ��ȡˮ�����ձ��н����ܽ⣬Ϊ�ٽ������ܽ⣬Ҫ�ò��������裬����Һ�ָ������º�ͨ������������ת����������ƿ�У�����ý�ͷ�ιܶ��ݡ�������ͼ��ʾ�����У�����������Һ�϶�����Ҫ���Ƿ�Һ©���������C������Ҫʹ�õIJ����������ձ�����������
(2)������500mL 0.2mol/LNaOH��Һ��Ҫ�������Ƶ�����Ϊ��m(NaOH)= 0.2mol/L��0.5L ��40g/mol=4.0g��
����������ƽ����NaOH���壻
����������ƿҪ�����Ƶ���Һ���¶������£�����Ҫ���ƺõ�NaOH��������ձ��У�����������ˮ�ܽ⡢���裬����ȴ�����£�
�ܽ�NaOH��Һ�ز�����ע��500mL����ƿ�У�
������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲ��ע������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�
������ˮע������ƿ��Һ����̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ���ڿ̶������У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ��͵õ�500mL 0.2mol/LNaOH��Һ��
(3)����ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L��С��0.2mol/L��
A.ʹ����ֽ����NaOH���壬������������NaOH����մ����ֽ�ϣ��������ʵ�����ƫ�٣���ȡ�����������������ʵ���Ҳ��ƫС��ʹ������Һ��Ũ��ƫ�ͣ�A�������⣻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ��������ʵ��������٣�ʹ������Һ��Ũ��ƫ�ͣ�B�������⣻
C.����ƿ��ԭ������������ˮ����Ӱ�����ʵ���������Һ���������˶����Ƶ���ҺŨ����Ӱ�죬C���������⣻
D.����ʱ���õ��������⣬ʹ���ʵ�����ƫ����������Ƶ���ҺŨ��ƫ�ߣ�D���������⣻
E.δ��ȴֱ��ת��������ƿ��������ã�����Һ�ָ�������ʱ����Һ�����ƫС���������Ƶ���ҺŨ��ƫ�ߣ�E���������⣻
�ʺ���ѡ����AB��


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijһ�������Է���мΪԭ���Ʊ�FeCl3��Һ������ӡˢ��·ͭ�帯ʴ����������ҺB���е�����ʵ��������ͼ��
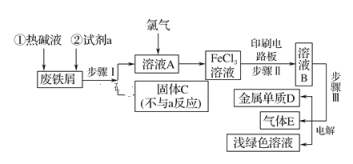
��1���ȼ�Һ������_____________��
��2���Լ�aӦѡ��________(��д����)��
��3���������õ�����Ҫ����������©����_____(��д��������)��
��4��д������������Ҫ��Ӧ�Ļ�ѧ����ʽ______��
��5��ʵ������ȡ����E�����ӷ���ʽ��____����������E���и�������գ���ѡ������װ���е�____(��д���)��
��6������û�ѧ������������E��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������ĩ����CaCO3��Na2SO4��KNO3��BaCl2��CuSO4�е�����������ɣ�ȡ��Ʒ������ͼʵ�飬��ʵ������жϣ���������

A.�ù����ĩ��һ��������BaCl2
B.�ù����ĩ��һ������KNO3
C.������ɿ�����CaCO3��BaCl2��Na2SO4
D.�������һ����CaCO3��Na2SO4��KNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У��������Ĵ������Ӧ���ͣ�������������ԭ��Ӧ����
A. 2Na+2H2O===2NaOH+H2��
B. Cl2+H2O===HCl+HClO
C. CaCO3![]() CaO+CO2��
CaO+CO2��
D. Ca��ClO��2+2HCl===CaCl2+2HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
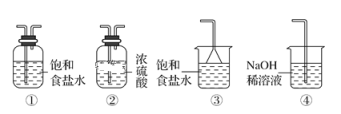
����Ŀ��ij��ѧ��ȤС���Ի�ͭ��(��Ҫ�ɷ�CuFeS2)Ϊԭ�Ͻ�������ʵ��̽����Ϊ�ⶨ��ͭ������Ԫ�ص�������������m1g�û�ͭ����Ʒ������ͼ��ʾװ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�ͭ����Ʒ��

��1����ƿA����ʢ�Լ���__________��װ��B��������__________����ƿD�ڷ�����Ӧ�����ӷ���ʽΪ__________��
��2����Ӧ��������ƿD�е���Һ�������´�����

��ͼ������ƿD�м������H2O2��Һ��Ӧ�����ӷ���ʽΪ__________����������ϴ�ӡ���ɡ����أ�����ϴ�ӵľ��巽��__________���û�ͭ������Ԫ�ص���������Ϊ__________(�ú�m1��m2�Ĵ���ʽ��ʾ)��
��3����Ӧ����徭���������պ�õ���ͭ(Cu��Cu2O)������(Fe2O3��FeO)��Ҫ��֤�����д���FeO��Ӧѡ�õ�����Լ���__________
A��KSCN��Һ����ˮB��ϡ���ᡢKMnO4��Һ
C��ϡ���ᡢKMnO4��ҺD��NaOH��Һ
��4����֪��Cu+��ǿ���Ի����лᷢ����Ӧ����Cu��Cu2+�����ʵ�鷽����֤��ͭ���Ƿ���Cu2O__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ��������6.0mol/L��H2SO4 1000 mL��ʵ���������ֲ�ͬŨ�ȵ����
��480mL 0.5mol/L �������150mL 25%������(��=1.18g/mL)����������18mol/L�����ᡣ�����ֹ�������ƿ��250mL��500mL��1000mL����ʦҪ����٢���������ȫ�����꣬����IJ������������䡣
��ش��������⣺
(1)ʵ������25%����������ʵ���Ũ��Ϊ______mol/L(����1λС��)��
(2)���Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ______mL��
(3)����ʱ����ͬѧ�IJ���˳�����£��뽫��������B��D����������
A�����٢�����Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ�����18mol/L��Ũ����____mL���ز����������������Һ�С����ò��������裬ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D��_________________________________________________________________________________________________________________________________
E��������������ƿ�м�ˮ��ֱ��Һ��ӽ��̶���1��2cm����
F�����ý�ͷ�ιܼ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶������У�
G��������ƿ�ǽ�����ҡ�ȡ�
(4)���ʡ�Բ���D����������ҺŨ���к�Ӱ�죿________(����ƫ��������ƫС��������Ӱ����)��
(5)���в���Cǰ����ע��____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�������л���������������������ţ��ɶ�����з��������Ԥ�⡣
�������л����������ڴ������ʵ���____(����ĸ)��
a��CH3CH2OH b��CH3CHO c��![]()
�������л��������м���Ũ��ˮ�����ɰ�ɫ��������____(����ĸ)��
a��CH2��CH2�� ������ b���� ������ c��![]()
�������л��������ܷ���ˮ�ⷴӦ����____(����ĸ)��
a�����ᡡ�� b����֬���� c��������
��2���л���W��![]() ���㷺�����������ֲ��Ĺ����С�
���㷺�����������ֲ��Ĺ����С�
��W���������������ŵ�����Ϊ____��____��
��W�����д���ͬһƽ���̼ԭ�������____����
��1mol W�����������____mol H2�����ӳɷ�Ӧ��
��3�����ǻ������������� ���dz��õ�ʳƷ����������ͨ�����·����ϳɣ�
���dz��õ�ʳƷ����������ͨ�����·����ϳɣ�
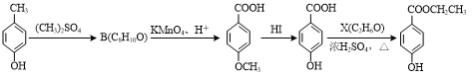
A���������������� ������������ C �������� D���������� E
��C��D�ķ�Ӧ����Ϊ____��
��B�Ľṹ��ʽΪ____��
��D��̼�����Ʒ�Ӧ�������л���Ľṹ��ʽΪ____��
��D��E��Ӧ�Ļ�ѧ����ʽΪ____��
��д��ͬʱ��������������C��һ��ͬ���칹��Ľṹ��ʽ��____��
���ܺ�FeCl3��Һ������ɫ��Ӧ��
���ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��
�����к���4�ֲ�ͬ��ѧ��������ԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС�����ʵ���Ʊ���������(��ͼ1)��
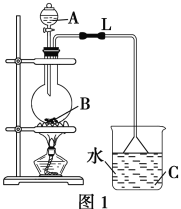
��1��ʵ������У��þƾ��ƻ������ȣ�������������Ŀ����______________________��
��2����ƿB�мӼ������Ƭ����������__________��������L��������________________��
��3��ͼ1����������ȱ�ݣ�������Ľ����飺_____________________________________�����Ľ���ʵ��������____________________________________________________�������������������IJ���������________________����Ҫ�õ�������������________(�����)��
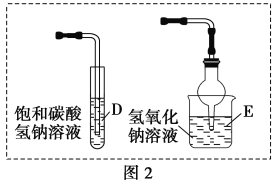
��4�����ܷ���ͼ2��Dװ�����ͼ1��Cװ�ã�________(����������������)��������__________________________��
���ܷ���ͼ2��Eװ�����ͼ1��Cװ�ã�________(����������������)��������___________________��
��5��ʵ���У���Ũ���������࣬�ᵼ�º����______________________________________(�����㼴��)����ʵ��֤�����������������ƹ������Ũ������ɱ�ʵ�飬�������Ʋ������л�����ŵ���____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��֪Ksp[Cu(OH)2]��2.2��10��20��Ksp[Fe(OH)3]��2.6��10��39�������£�ij����CuCl2��Һ�к���������FeCl3��Ϊ�˵õ�������CuCl2��2H2O���壬Ӧ����___________(��������Ļ�ѧʽ)��������Һ��pH��4��ʹ��Һ�е�Fe3��ת��ΪFe(OH)3��������ʱ��Һ�е�c(Fe3��)��________�����˺�������Һ����������Ũ���ᾧ���ɵõ�CuCl2��2H2O���塣
(2)ij̼�ظֹ�¯��ˮ������Ҫ�ɷ���̼��ơ�����ơ�������þ�����⡢��������ȡ�ˮ���輰ʱ��ϴ��ȥ����ϴ�������£�
��.����NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
��.�ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
��.��ϴ��Һ�м���Na2SO3��Һ��
��.��ϴ��꣬��NaNO2��Һ�ۻ���¯��
����ϡ�����ܽ�̼��Ƶ����ӷ���ʽ��_____________________________��
����֪��25 ��ʱ�й����ʵ��ܶȻ�
���� | CaCO3 | CaSO4 | Mg(OH)2 | MgCO3 |
Ksp | 2.8��10��9 | 9.1��10��6 | 1.8��10��11 | 6.8��10��6 |
�������ݣ���ϻ�ѧƽ��ԭ��������ϴCaSO4�Ĺ���________________�������ܽ�ƽ�����ʽ�ͱ�Ҫ��������������˵�������ڲ������ݹ����л��ᷢ����ӦMgCO3(s)��2OH��(aq)![]() Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
Mg(OH)2(s)��CO32-(aq)���÷�Ӧ��ƽ�ⳣ��K��________(������λ��Ч����)��
�۲�����У�����Na2SO3��Һ��Ŀ����_______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com