
���������ѧУ��ѧʵ����װ�ޣ�ҩƷ��ת�Ƶ��˰�ȫ�ij������ܣ�������Ϊ������Ա��������м�ƿ��������û�м�ʱת�ƣ���ѧ��ŷ��֣�������Ҫ�ⶨ���������Ƿ��б��ʣ������ʲô������ij��ȤС�������������������ô���ģ������£���ȡ��ͬ����������Ʒ����ˮ���������к���pH=7����Ӧ������û�й۲쵽���ݣ�Ȼ����Һ���ɵ��Ȼ��ƾ��壬���ɹ����в�Ʒ����ʧ��
| | ������������(g) | �Ȼ�������(g) |
| �� | 2.40 | 3.51 |
| �� | 2.32 | 2.34 |
| �� | 3.48 | 3.51 |
��һ��Ϊ�������� �ڶ��顢�����鶼Ϊһˮ����������
��������������������ᣬ��Ӧ������û�й۲쵽���ݣ�˵������Na2CO3��NaHCO3��
NaCl�����ʵ���Ϊ��3.51g��58.5g/mol=0.06mol��������Ԫ���غ㣬NaOH�����ʵ���Ϊ��0.06mol����Ʒ��Ħ������Ϊ��2.40g��0.06mol=40g/mol��������ƷΪNaOH��
NaCl�����ʵ���Ϊ��2.34g��58.5g/mol=0.04mol��������Ԫ���غ㣬NaOH�����ʵ���Ϊ��0.04mol����Ʒ��Ħ������Ϊ��2.32g��0.04mol=58g/mol��������ƷΪNaOH?H2O��
NaCl�����ʵ���Ϊ��3.51g��58.5g/mol=0.06mol��������Ԫ���غ㣬NaOH�����ʵ���Ϊ��0.06mol����Ʒ��Ħ������Ϊ��3.48g��0.06mol58g/mol��������ƷΪNaOH?H2O��
���㣺���⿼�黯ѧ���㡢Ԫ���غ㡣


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����������ܵ������ ������ţ���ͬ�������ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��ˮ�� ��CuSO4?5H2O �۴���ʯ ���Ȼ��ƾ��� ������ �ް���
������ ������ ��Һ̬�Ȼ��� ��������Һ
��2����Ҫ��ش��������⡣
��Al2��SO4��3�ĵ��뷽��ʽ��
�� NaHCO3�ĵ��뷽��ʽ��
���û�ѧ����ʽ˵��������Ʒ�ĩ�����ܷⱣ���ԭ��
��д��������ˮ��Ӧ�����ӷ���ʽ��
��3����Ҫ�����������գ�
����ͬ���������������顢ˮ�����к������������� ��
��4.9 g H2SO4������ ��ԭ�ӡ�
��a��Xԭ�ӵ�������Ϊb g����X�����ԭ�������ɱ�ʾΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�������������棬��2011��5��1���𣬽�ֹ��������������ӹ������ƣ�CaO2����ʳƷ���Ӽ����������ƣ�CaO2����һ�ְ�ȫ�����ʣ����нᾧˮ��ͨ��������CaO��
��1����ȡ5.42 g����������Ʒ������ʱ�������·�Ӧ��
2��CaO2��xH2O�� 2CaO+O2��+2xH2O���õ�O2�ڱ�״�������Ϊ672 mL������Ʒ��CaO2�����ʵ���Ϊ_____��
2CaO+O2��+2xH2O���õ�O2�ڱ�״�������Ϊ672 mL������Ʒ��CaO2�����ʵ���Ϊ_____��
��2����ȡͬһ��Ʒ5.42 g����������ϡ�����У�Ȼ�����������Na2CO3��Һ������Һ��Ca2+ȫ��ת��ΪCaCO3�������õ������CaCO3 7.0 g��
����Ʒ��CaO������Ϊ_____��
����Ʒ��CaO2��xH2O��xֵΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣����ⶨij��������Ҫ�ɷ�������������������̼������ɣ�����������ʵ�飺��ȡ��ĩ״��Ʒ8.50�ˣ�����ijŨ�ȵ�����100 mL ����ַ�Ӧ���ռ�����״��������2.24 L ��Ȼ��������Ʒ�м���ͬŨ�ȵ�����100mL ����ַ�Ӧ�����ռ�����״��������1.12 L ��
����д�����������̣�
��1����ȡ��������ʵ���Ũ�ȡ���2����������Ʒ��̼����������������3λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ͬѧ�о��ӿ�ʯ��ʼ�����������Ĺ������̡�
��1����6.62g����ʯ��ƷͶ����������������ȫ�ܽ����ˣ������в�����Ԫ�ء�����Һ�мӹ�����NaOH��Һ����ַ�Ӧ�����ˡ�ϴ�ӡ����յ�4.80g Fe2O3���������ʯ����������������
��2�����Ը�����ʯΪԭ����������������������Ԫ����ʧ4��������ÿ����1.00������������96������������Ҫ��������ʯ���ٶ�?��������λС����
��3��ȡij������ĩ28.12g������ֻ��Fe��C�������������г�ַ�Ӧ���õ�CO2����224mL����״����������˸�����ĩ������̼�����ʵ���֮�ȡ�
��4����ȡ���ݲ�ͬ����������������ĩ���ֱ�ӵ�100mL��ͬŨ�ȵ�H2SO4��Һ�У���ַ�Ӧ��õ�ʵ���������±���ʾ��
| ʵ����� | �� | �� | �� |
| ���������ĩ������/g | 2.812 | 5.624 | 8.436 |
| ������������/L����״���� | 1.120 | 2.240 | 2.800 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��֪һ������������������ȼ�գ����û������100 mL 3.00 mol/L��NaOH��Һ���ܶ�Ϊ1.12 g/mL��ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.0500 mol��
��1��ԭNaOH��Һ����������Ϊ_______
��2��������Һ��Cl�������ʵ���Ϊ________mol
��3�����������Ͳμӷ�Ӧ�����������ʵ���֮��n(Cl2)��n(H2)��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ������PH3��H2�Ļ�����壬����ͨ����֧���ȵ�Ӳ�ʲ����ܣ���һ֧װ��������ͭм���ڶ�֧װ��������CuO����һ֧��������������Ӧ��2PH3��3Cu Cu3P2��3H2������4.96 g���ڶ�֧�����ܷ�Ӧ������������5.76 g��
Cu3P2��3H2������4.96 g���ڶ�֧�����ܷ�Ӧ������������5.76 g��
����ԭ���������PH3��H2������ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ƽ��ÿ�����Լ600 m3�ϰ�ˮ��NH3��Ũ��Ϊ153 mg��L-1���ܶ�Ϊ1 g��cm��3��
��1���÷ϰ�ˮ�а������ʵ���Ũ��Ϊ ��
��2���Էϰ�ˮ���м��������õ�NH3��ʹ�ϰ�ˮ�е�NH3 ��Ũ�Ƚ�Ϊ17 mg��L-1����������ǰ��ϰ�ˮ����仯������������ķϰ�ˮ����������NH3�����ʵ���Ϊ ��(���¼�������������λС��)
��3�������õ���NH3�������Ʊ�NO��4NH3+5O2 ��4NO+6H2O;��������NO�������ķ�Ӧ������������������������Ϊ0.20�������������Ϊ0.80��
��ΪʹNH3ǡ����ȫ����ΪNO����������������а����������Ϊ________��
�ڰ���������ȡNOͬʱ�ᷢ������Ӧ��4NH3+3O2 ��2N2+6H2O����1L NH3���10L������ͨ�뷴Ӧ������Ӧ��ɺ�û�������в���NH3����O2��N2�����ʵ���֮��Ϊ1��10������μ�����Ӧ�İ�ռԭ�ϰ�������ٷֺ�����
��4���ϰ�ˮ�������ѳ������е�SO2����ˮ����������SO2������������(NH4)2SO4��NH4HSO4�����ֳ�ȡ��ͬ�����Ļ�������ʵ�飬������£�
| NaOH ��Һ���/mL | 40.00 | 40.00 | 40.00 |
| ��Ʒ����/g | 7.75 | 15.50 | 23.25 |
| ��������/g | 1.87 | 1.87 | 1.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijһ��θҩ�е������Ϊ̼���,��������������������IJⶨ����:
��������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ
��ȡһ��(ҩƬ������ͬ)0.2 g�Ĵ�θҩƬ,ĥ������20.0 mL����ˮ
���Է�̪Ϊָʾ��,��0.1 mol��L-1��NaOH��Һ�ζ�,��ȥV mL��ζ��յ�
�ܼ���25 mL 0.1 mol��L-1��HCl��Һ
(1)д��ʵ����̵IJ���(д���˳��)������������������������
(2)��ͼ��ʾ����������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ�϶�����Ҫ��������(�����)������,����������Һ����Ҫ�IJ���������������(����������)�� 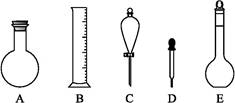
(3)����������ҺӦѡ�õ�����ƿ�����(����ĸ)������������
| A��50 mL��50 mL |
| B��100 mL��100 mL |
| C��100 mL��150 mL |
| D��250 mL��250 mL |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com