
��1�����������ܵ������ ������ţ���ͬ�������ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��ˮ�� ��CuSO4?5H2O �۴���ʯ ���Ȼ��ƾ��� ������ �ް���
������ ������ ��Һ̬�Ȼ��� ��������Һ
��2����Ҫ��ش��������⡣
��Al2��SO4��3�ĵ��뷽��ʽ��
�� NaHCO3�ĵ��뷽��ʽ��
���û�ѧ����ʽ˵��������Ʒ�ĩ�����ܷⱣ���ԭ��
��д��������ˮ��Ӧ�����ӷ���ʽ��
��3����Ҫ�����������գ�
����ͬ���������������顢ˮ�����к������������� ��
��4.9 g H2SO4������ ��ԭ�ӡ�
��a��Xԭ�ӵ�������Ϊb g����X�����ԭ�������ɱ�ʾΪ________��
��1���٢ݢ⣻�ڢۢܢ�ޢ� ��2����Al2��SO4��3=2Al3++3SO42-
��NaHCO3=Na++HCO3- ��Ca(ClO)2+CO2+H2O=CaCO3+2 HClO
(��д��ʽҲ�ɵ�����2HClO 2 HCl+O2��) ��Cl2+H2O
2 HCl+O2��) ��Cl2+H2O  H++Cl-+HClO���á�=�����۷֣�
H++Cl-+HClO���á�=�����۷֣�
��3������������0. 35 NA ����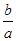 NA��
NA��
���������������1���٢��ǵ��ʣ����ǽ������ڽ����к��������ƶ��ĵ��ӿ��Ե��磬���Ƿǽ�������û�������ƶ��ĵ��ӻ����ӣ����ܵ��磻�ڢۢ��������ڵ���ʣ������ᣬҲ�ǵ���ʣ����Тڢۢ��京�����ӣ������ڲ��������ƶ����ʲ����Ե��磻��Һ̬�Ȼ����ǹ��ۻ���������� �����ܵ��硣�ݢ��ǻ��������������ƶ������ӣ������磻�ޢ��Ƿǵ���ʣ��������ƶ������ӣ����ܵ��硣 �����������ܵ���������Ǣ٢ݢ⣬���ڵ���ʵ��Ǣڢۢܢᣬ���ڷǵ���ʵ��Ǣޢߡ���2����Al2��SO4��3���Σ�����ǿ����ʣ����ĵ��뷽��ʽ�ǣ�Al2��SO4��3=2Al3++3SO42-�� NaHCO3���������ʽ�Σ�����д���뷽��ʽʱ��������Ӳ��ܲ����ĵ��뷽��ʽ�ǣ�NaHCO3=Na++HCO3-���۴����������ᣬ���Ա�̼�ỹ����������������ڷ��ã���Ϳ����еĶ�����̼��ˮ������Ӧ��Ca(ClO)2+CO2+H2O=CaCO3+2 HClO ������ʧЧ����Ҫ�ܷⱣ�档��������ˮ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�������ӷ���ʽ��Cl2+H2O  H++Cl-+HClO��3��n=m�MM,n=N�MNA,��ͬ����ʱ���������ʵĵ�Ħ������ԽС�������ʵ�����Խ��������Խ�ࡣ������������������Ħ��������С�����ķ�����Ҳ����ࡣ��������n=m�MM,��������ӵ����ʵ�����������,n=N�MNA���������������ÿ�������к���7��ԭ�ӣ�������������7���͵õ���Ӧ��ԭ��������1��Xԭ�ӵ�����Ϊb�Mag����NA��ԭ�ӵ�������ΪNAb�Mag����Ħ������ΪNAb�Mag�Mmol��X�����ԭ����������ֵ�ϵ�����Ħ��������ΪNAb �Ma��
H++Cl-+HClO��3��n=m�MM,n=N�MNA,��ͬ����ʱ���������ʵĵ�Ħ������ԽС�������ʵ�����Խ��������Խ�ࡣ������������������Ħ��������С�����ķ�����Ҳ����ࡣ��������n=m�MM,��������ӵ����ʵ�����������,n=N�MNA���������������ÿ�������к���7��ԭ�ӣ�������������7���͵õ���Ӧ��ԭ��������1��Xԭ�ӵ�����Ϊb�Mag����NA��ԭ�ӵ�������ΪNAb�Mag����Ħ������ΪNAb�Mag�Mmol��X�����ԭ����������ֵ�ϵ�����Ħ��������ΪNAb �Ma��
���㣺�������ʡ��ǵ���ʵȸ�����ʵĵ�������֮�Ĺ�ϵ�����ʵ��������������ʵ������Ĺ�ϵ��Ħ�����������ԭ�������Ĺ�ϵ��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
��1����Ҫ98%�ܶ�Ϊ1.84g/cm3��Ũ���� mL
��2������ʱ������ʹ�õ������� ���������� (�����) ��ȱ�ٵ������� ������ ��
���ձ�����100 mL��Ͳ������20 mL��Ͳ ��1000 mL����ƿ ��250 mL����ƿ����������ƽ(������) �߲�����
��3������ʱ����ʵ�������õ��������������÷ֱ��� �� ��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ���ƫ�͡�����Ӱ�족��
��û��ϴ���ձ��Ͳ���������������������������
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶��ߡ�����������������
������ƿû�и������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��2.3����Ͷ�뵽ˮ�����з�̪���У���Ӧ��������Һ��0.1������
��1����Ӧ�����У����Է����Ƹ���ˮ�棬˵���Ƶ��ܶ� ������ڡ���С�ڡ�����
�ڡ���ˮ���ܶȣ���Һ����� ɫ��
��2����ѧ��Ӧ����ʽ�� ��
��3���������Һ�����ʵ���Ũ���Ƕ��٣���д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺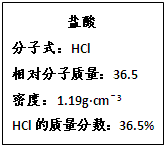
(1)��Ũ������HCl�����ʵ���Ũ��Ϊ___________������KMnO4����������HCl���䷴Ӧ����ʽ���£�
2KMnO4+16HCl(Ũ)��2KCl+2MnCl2+5Cl2��+8H2O
(2)1molKMnO4��ȫ��Ӧ����Ӧ����Һ�����Ϊ2L�������ò�����Cl�������ʵ���Ũ��Ϊ____________��
(3)8molHCI��ȫ��Ӧ��������Cl2�ڱ�״���µ����Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ��12.5 mol/LŨ����������500 mL1 mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��й����⣺
��1����Ҫ��ȡ12.5 mol/L��ŨHNO3________mL�����Ƹ�ϡ����ʱʹ�õ���������Ͳ�����������ձ��⣬�������õ���������______��___________�ȡ�
��2��ȡ�����Ƶ�ϡHNO3200 mL����һ��������ͭ�۳�ַ�Ӧ��ͭ��ȫ���ܽ�����ɵ�Ψһ��ԭ����NO�����ڱ���µ����Ϊ0.56L����д��Cu��ϡHNO3��Ӧ�Ļ�ѧ����ʽ ���μӷ�Ӧ��ͭ�۵�����Ϊ_________g��
��3����Ҫ����Һ��Cu2+ȫ��ת��ΪCu(OH)2���������������0.2mol/L��NaOH��Һ�����Ϊ ml.
��ע��Ҫ���ڴ�������淽���ڽ��г��������ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ���ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Al3+��K+�е����ֻ���֡�ȡ����Һ100mL���������NaOH��Һ�����ȣ��õ�448mL(���)���壬ͬʱ�������ɫ�����������ˡ�ϴ�ӡ����գ��õ�1.6g���壻��������Һƽ���ֳ����ݣ�һ���м�����BaCl2��Һ���õ�2.33g����������ij�������һ����ͨ�����CO2�õ�1.56g��ɫ�������ɴ˿��ƶ�ԭ��Һһ�����е��������༰��Ũ�ȣ�����������±����ɲ���������
| һ�����е��������� | | | | | | | |
| ���ʵ���Ũ��(mol/L) | | | | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Իش��������⣺
��1����֪24��A��40��Bǡ����ȫ��Ӧ����0.4molC��32��D����C��Ħ������Ϊ ��
��2����ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ mol/L��
����ʵ��������450mL1.19 mol/L��ϡ���ᣬ���ø�Ũ����________ mL,����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������_______________(����������)��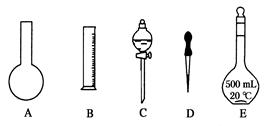
��ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��____1.19mol/L (����ڡ������ڡ���С�ڡ�����ͬ)����������Һ��ת��������ƿʱ����������������������ҺŨ��_______1.19mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
д���������ʵĵ���ʽ���нṹʽ����д���ṹʽ
�ٰ��� �� ���Ȼ�� ��
�۸ɱ� �� �ܿ����� ��
�ݱ� �� �������� ��
��˫��ˮ �� ��HClO ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���������ѧУ��ѧʵ����װ�ޣ�ҩƷ��ת�Ƶ��˰�ȫ�ij������ܣ�������Ϊ������Ա��������м�ƿ��������û�м�ʱת�ƣ���ѧ��ŷ��֣�������Ҫ�ⶨ���������Ƿ��б��ʣ������ʲô������ij��ȤС�������������������ô���ģ������£���ȡ��ͬ����������Ʒ����ˮ���������к���pH=7����Ӧ������û�й۲쵽���ݣ�Ȼ����Һ���ɵ��Ȼ��ƾ��壬���ɹ����в�Ʒ����ʧ��
| | ������������(g) | �Ȼ�������(g) |
| �� | 2.40 | 3.51 |
| �� | 2.32 | 2.34 |
| �� | 3.48 | 3.51 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com