
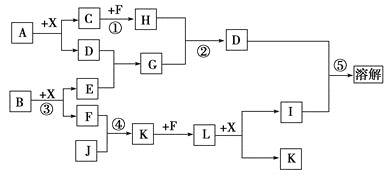
����Ŀ����֪A��B����������Ԫ����ɵĻ����A��ij��Ԫ�ص���������Ϊ![]() ��B��һ�ֵ���ɫ���壬C��J��ͬ����Ԫ�ص���̬�⻯�����C�Ǻ�������ߵ�����DΪ��������ǿ����������ǿ��İ�ɫ��״���ʣ�XΪ��������ɫҺ�塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��
��B��һ�ֵ���ɫ���壬C��J��ͬ����Ԫ�ص���̬�⻯�����C�Ǻ�������ߵ�����DΪ��������ǿ����������ǿ��İ�ɫ��״���ʣ�XΪ��������ɫҺ�塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��
(1)д��A�Ļ�ѧʽ��________
(2)д�� B�ĵ���ʽ______________
(3)��Ӧ![]() ��ÿ����
��ÿ����![]() ��ת�Ƶ��ӵ���ĿΪ________
��ת�Ƶ��ӵ���ĿΪ________![]() ��
��![]() ��ʾ�����ӵ�����
��ʾ�����ӵ�����![]()
(4)��Ӧ![]() �Ļ�ѧ����ʽΪ��______________________________
�Ļ�ѧ����ʽΪ��______________________________
(5)д��H����ʱ��Ӧ![]() �����ӷ���ʽ_______________________________________
�����ӷ���ʽ_______________________________________
���𰸡�![]()
![]()
![]() 4NH3+5O2
4NH3+5O2![]() 4NO+6H2O
4NO+6H2O ![]()
��������
������Ŀ������Ϣ������ɫ���壬��������ߵ����Ʋ�����ʣ�������Щ���ʵ����ʣ��ƶϳ��������ʵ����ʣ���д����ѧ����ʽ�����ӷ���ʽ��
C�Ǻ�������ߵ�������Ϊ���飻DΪ��������ǿ����������ǿ��İ�ɫ��״���ʣ�Ϊ����������B�ǵ���ɫ���壬��B����X��Ӧ����E��F����BΪ![]() ��EΪNaOH��FΪ
��EΪNaOH��FΪ![]() ��XΪ
��XΪ![]() ��D(��������)����E��Ӧ����G����GΪƫ�����ƣ�C��F��Ӧ����H����HΪ������̼����̬�⻯��J��F(����
��D(��������)����E��Ӧ����G����GΪƫ�����ƣ�C��F��Ӧ����H����HΪ������̼����̬�⻯��J��F(����![]() ��Ӧ�õ�K��K��F(����)��Ӧ�õ�L��L��X(ˮ)��Ӧ�õ�I��K����ѧ�й�ҵ�Ʊ��������ת����ϵ������֪JΪ
��Ӧ�õ�K��K��F(����)��Ӧ�õ�L��L��X(ˮ)��Ӧ�õ�I��K����ѧ�й�ҵ�Ʊ��������ת����ϵ������֪JΪ![]() ��KΪNO��LΪ
��KΪNO��LΪ![]() ��IΪ
��IΪ![]() ��CΪ
��CΪ![]() ��DΪ������������A�к���C��AlԪ�أ���AΪ
��DΪ������������A�к���C��AlԪ�أ���AΪ![]() ��������Ԫ����������Ϊ
��������Ԫ����������Ϊ![]() ���������⡣
���������⡣
(1)������������֪��AΪ![]() ��
��
(2)![]() �ǹ������ƣ�����ʽΪ
�ǹ������ƣ�����ʽΪ![]() ��
��
(3)��Ӧ![]() ��ˮ��Ӧ�����������ƺ�������2Na2O2+2H2O=4NaOH+O2����������������Ԫ�صĻ��ϼ۴�-1���͵�-2���ĸ���ԭ�ӣ�������ԭ�Ӵ�-1���ߵ�0�ۣ�����������ԭ�Ӵ�-1�۽��͵�-2�ۣ�ÿ����
��ˮ��Ӧ�����������ƺ�������2Na2O2+2H2O=4NaOH+O2����������������Ԫ�صĻ��ϼ۴�-1���͵�-2���ĸ���ԭ�ӣ�������ԭ�Ӵ�-1���ߵ�0�ۣ�����������ԭ�Ӵ�-1�۽��͵�-2�ۣ�ÿ����![]() ��ת�Ƶ���2mol��ת�Ƶ��ӵ���ĿΪ
��ת�Ƶ���2mol��ת�Ƶ��ӵ���ĿΪ![]() ��
��
(4)��Ӧ![]() Ϊ�����Ĵ���������ѧ����ʽΪ��4NH3+5O2
Ϊ�����Ĵ���������ѧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
(5)��Ӧ![]() Ϊ
Ϊ![]() ��
��![]() �ķ�Ӧ��
�ķ�Ӧ��![]() ����ʱ�����߷�Ӧ��������������̼��������ӷ���ʽΪ��
����ʱ�����߷�Ӧ��������������̼��������ӷ���ʽΪ��![]() ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�л���A��B��C��D��E��F��G������ת����ϵ������C�IJ�������������һ�����ҵ�ʯ�ͻ�����չˮƽ��G�ķ���ʽΪC9H10O2���Իش������й����⣺
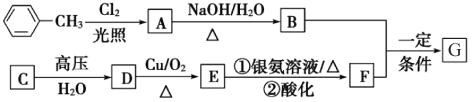
��ָ�����з�Ӧ�ķ�Ӧ���ͣ�Cת��ΪD��_________��
��д��A�й����ŵ����ƣ�_________��
��д�����з�Ӧ�Ļ�ѧ����ʽ��
D����E�Ļ�ѧ����ʽ��_________��E��������Һ��Ӧ�Ļ�ѧ����ʽ_________��B��F����G�Ļ�ѧ����ʽ��_________��
��д����������������G��ͬ���칹��Ľṹ��ʽ��_________��
������FeCl3������ɫ��Ӧ
���������Ƶ�������Һ��Ӧ��������������
���˴Ź���������ʾ�����ֲ�ͬ��ѧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ���۽�Ϊ������û�ѧʵ�����������ۣ����ʹ���۾���Ȥζ�ԣ�ij�༶��ѧѧϰС��ͨ��ʵ�����Ʊ�CO2�ķ�Ӧ̽��ijЩ��ѧ���ۡ��±���ʵ������е����ݼ������Ϣ��
��� | ��Ӧ �¶�/�� | c(HCl)/(mol��L-1) | V(HCl)/mL | 10g CaCO3 ����״ | t/min |
�� | 20 | 2 | 10 | ��״ | t1 |
�� | 20 | 4 | 10 | ��״ | t2 |
�� | 20 | 2 | 10 | ��״ | t3 |
�� | 40 | 2 | 10 | ��״ | t4 |
�� | 40 | 4 | 10 | ��״ | t5 |
![]() ��ʾ�ռ�CO2���Ϊa mL�����ʱ�䡣ע���������������ͬ�����²�á�
��ʾ�ռ�CO2���Ϊa mL�����ʱ�䡣ע���������������ͬ�����²�á�
(1)�ɱ��е���Ϣ��֪��ʵ���Ŀ����̽��__________��
(2)�����е�ʵ��ٺ�ʵ�����̽��_____�Ի�ѧ��Ӧ���ʵ�Ӱ�졣���������е���Ϣ��֪��Ӱ��û�ѧ��Ӧ���ʵ����ػ���______________________________��
(3)�ռ�a mLCO2�����ʱ�����ٵ���ʵ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������A������Ԫ����ɣ�ij��ȤС�����������ʵ�飺

��֪�������Ϊ��������ֻ������Ԫ�أ��ڱ�������Ϊ672 mL����Һ��Ϊ������ҵ�г��õ�ճ�ϼ���
��ش��������⣺
��1��A�����Ԫ��Ϊ________����Ԫ�ط��ű�ʾ����
��2��д���������NaOH(aq)��Ӧ�����ӷ���ʽ________��
��3�������£�A�����������ܷ������ұ�ը���������ֳ�����������д����Ӧ�Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦmX(g)![]() nY(g)��pZ(g) ��H���ڲ�ͬ�¶��µ�ƽ����ϵ������Y�����������ѹǿ�仯��������ͼ��ʾ������˵���������( )
nY(g)��pZ(g) ��H���ڲ�ͬ�¶��µ�ƽ����ϵ������Y�����������ѹǿ�仯��������ͼ��ʾ������˵���������( )

A. �÷�Ӧ����H��0
B. m��n��p
C. B��C���㻯ѧƽ�ⳣ����KB��KC
D. A��C����ķ�Ӧ����v(A)��v(C)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2��һ�����������������������Ƴ�NaClO2���壬�Ա���������棬�������ⷨ��NaClO2�����ʵ��װ����ͼ��ʾ��
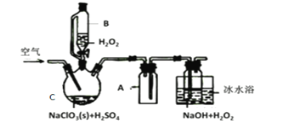
��֪����2NaC1O3+H2O2+H2SO4=2C1O2��+O2��+Na2SO4+2H2O
2ClO2+H2O2+2NaOH=2NaClO2+O2��+2H2O
��ClO2�۵�-59�桢�е�11�棬Ũ�ȹ���ʱ�����ֽ⣻
��H2O2�е�150��
��1����ˮԡ��ȴ��Ŀ����___��
��2���������ٹ���������������NaClO2���ʣ��Խ�����ԭ�������ٹ���ʱ��__��
��3��Cl-����ʱ���ClO2�����ɡ���Ӧ��ʼʱ��C�м����������ᣬClO2���������ʴ����ߣ����������������ù��̿��ܾ�������ɣ��뽫�䲹��������
��___�������ӷ���ʽ��ʾ����H2O2+Cl2=2Cl-+O2+2H+
��4��NaClO2���Ȳⶨ��
��ȷ��ȡ����NaClO2��Ʒ10.0g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ��C1O2-�IJ���ΪCl-���������û��Һ���250mL������Һ��
��ȡ25.00mL����Һ����2.0mol��L-1Na2S2O3��Һ�ζ�(I2+2S2O32-=2I-+S4O62-)���Ե�����Һ��ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ__���ظ��ζ�3�Σ����Na2S2O3��Һƽ������Ϊ20.00mL�������Ʒ��NaClO2����������Ϊ___����M(NaClO2)=90.5g/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������ĵ���ƽ�ⳣ�����£�
��ѧʽ | HF | HCN | H2CO3 |
���볣�� | Ka=3.5��10-4 | Ka=5.0��10-10 | Ka1=4.4��10-7 Ka2=4.7��10-11 |
��1��c(H+)��ͬ����������Һ��Ũ�ȴӴ�СΪ___��
��2����HCN��Һ����ʼŨ��Ϊ0.01mol��L-1��ƽ��ʱc(H+)ԼΪ__mol��L-1��ʹ����Һ��HCN�ĵ���̶�������c(H+)Ҳ����ķ�����__��
��3���к͵�����NaOH�����ĵ�pH�����������������ֱ�ΪaL��bL����a__(������������С������������������ͬ)b���к͵�Ũ�ȡ��������������������ҪNaOH�����ʵ���Ϊn1��n2����n1__n2��
��4����NaCN��Һ��ͨ��������CO2��������Ӧ�����ӷ���ʽΪ__��
��5�����ʵ��֤��������HCl��������__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������X������Ԫ����ɣ�Ϊ̽������ɵ����ʣ���Ʋ��������ʵ�飺
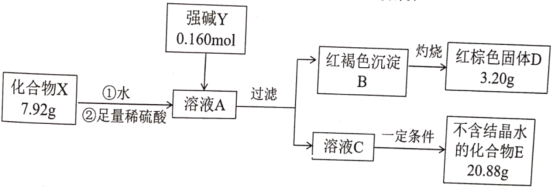
��ʾ��������E����ɫ��ӦΪ��ɫ������ɫ�ܲ�����
��ش�
��1��X�Ļ�ѧʽ��________________��ǿ��Y�ĵ���ʽΪ________________��
��2���ڳ��º���������£�������X�����ȶ����ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������һ�����嵥�ʡ�
�ٻ�����X��ˮ��Ӧ�����ӷ���ʽΪ________________��
��������Ի�����X���ȶ��Խ����˴������о�����ȡ����һ���Ľ�չ���������ʿ����������X��ˮ��Һ���ȶ��Ե���________________��
A KHSO4 B K2CO3 C CH3COOK D K2SO3
��Ϊ�о��¶ȶԻ�����Xˮ��Һ�ȶ��Ե�Ӱ�죬�����һ��ʵ�鷽����________________________________________________��
��3��������X�ж����Ʊ���������һ�ַ�������ǿ��Y�������ô����������ɫ����B��Ӧ���仯ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���а������ʣ������顢���������۱�ϩ�����������ϩ����2һ��Ȳ���������� ���ڶ��ױ����ѽ���������ʹ����KMnO4��Һ��ɫ��������ˮ��Ӧʹ֮��ɫ����( )
A.�ۢܢݢߢ�B.�ܢݢ�C.�ܢݢߢ�D.�ۢܢݢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com