
ЁОЬтФПЁПХ№ЧтЛЏФЦ(NaBH4ЃЌХ№ЮЊ+3Мл)ЮЊАзЩЋЗлФЉЃЌдкИЩдяПеЦјжаЮШЖЈЃЌдкГБЪЊПеЦјжаЗжНтЃЌЪЧГЃгУЕФЛЙдМСЁЃЦЋХ№ЫсФЦЃЈNaBO2ЃЉвзШмгкЫЎЃЌВЛШмгкввДМЃЌвзЫЎНтЁЃФПЧАгаЖржжЙЄвеПЩжЦБИNaBH4ЁЃ
ЃЈ1ЃЉгУХ№ОЋПѓЃЈКЌгавЛЖЈСПB2O3ЃЌМАAl2O3ЁЂSiO2ЁЂFe2O3ЕШдгжЪЃЉжЦШЁNaBH4ЕФСїГЬШчЯТЃК

ЂйЁАШмНтЁБЪБЃЌB2O3гыNaOHЗДгІЩњГЩСЫNaBO2ЃЌЗДгІРызгЗНГЬЪНЮЊ____ЁЃ
ЂкЁАГ§ЙшТСЁБВНжшМгШыCaOЃЌЖјВЛМгШыCaCl2ЕФдвђгаЃКФмНЋЙшЁЂТСвдГСЕэГ§ШЅЃЛОЁСПВЛДјШыдгжЪРызгЃЛ___ЁЃ
ЂлЁАВйзї2ЁБЪЧНЋТЫвКеєЗЂЁЂНсОЇЁЂЯДЕгЃЌЦфжаЯДЕгбЁгУЕФЪдМСзюКУЪЧ_____ЃЈЬюзжФИЃЉЁЃ
a. РфЫЎЁЁЁЁ b. ввДМЁЁ c. АБЫЎЁЁЁЁ d. NaOHШмвК
ЂмЁАЗДгІ1ЁБЪЧMgH2гыNaBO2ЛьКЯЕУЕНNaBH4КЭMgOЃЌЦфЛЏбЇЗНГЬЪНЮЊ________ЁЃ
ЃЈ2ЃЉЮвЙњЫябхЦНВЩгУФЭИЏЪДЕчМЋВФСЯЃЌвдбєРызгНЛЛЛФЄЮЊИєРыФЄЃЌЕчНтЦЋХ№ЫсФЦЕФМюШмвКЃЌвВПЩвдИпаЇжЦБИNaBH4ЁЃИУЙЄвебєМЋВњЮяЮЊ________ЃЌвѕМЋЕчМЋЗНГЬЪНЮЊ_____ЁЃ
ЁОД№АИЁПB2O3+2OH- =2BO2-+H2O ЪЙШмвКГЪМюадЃЌвжжЦNaBO2 ЕФЫЎНт b 2MgH2+NaBO2 =NaBH4+2MgO O2 BO2-+8e-+6H2O=BH4-+8OH-
ЁОНтЮіЁП
ЃЈ1ЃЉСїГЬХ№ОЋПѓжаМгШыЧтбѕЛЏФЦЗЂЩњЗДгІЃКB2O3+2NaOH =2NaBO2+H2OЃЌAl2O3+2NaOH=2NaAlO2+H2OЃЌSiO2+2NaOH=Na2SiO3+H2OЃЌЙ§ТЫЁЃГ§ЕєТЫдќFe2O3ЃЌТЫвКжаМгШыCaOЃЌЩњГЩNa2OЁЄ3CaOЁЄAl2O3ЁЄnSiO2ГСЕэ,Й§ТЫГ§ЕєТЫдќЃЌЭЌЪБМюадЛЗОГвжжЦNaBO2ЕФЫЎНтЃЌТЫвКеєЗЂЁЂНсОЇЁЂЯДЕгЃЌЕУЕНNaBO2ЃЌNaBO2КЭMgH2ЗДгІЩњГЩNaBH4ЁЃ
NaBO2КЭMgH2ЩњГЩNaBH4КЭбѕЛЏУОГСЕэЃЛ
ЃЈ2ЃЉИљОнЕчНтГибєМЋбѕЛЏЃЌЪЇЕчзгЃЌвѕМЋЛЙдЕУЕчзгЃЌХаЖЯбєМЋВњЮяЃЌЪщаДЕчМЋЗДгІЪНЁЃ
ЃЈ1ЃЉЂйB2O3гыNaOHЗДгІЩњГЩСЫNaBO2КЭЫЎЃЌЗДгІРызгЗНГЬЪНЮЊB2O3+2OH- =2BO2-+H2OЃЛ
Д№АИЃКB2O3+2OH- =2BO2-+H2O
ЂкЁАГ§ЙшТСЁБВНжшМгШыCaOЃЌCaO+H2O=Ca(OH)2,ШмвКГЪМюадЃЌПЩвдвжжЦNaBO2 ЕФЫЎНтЃЛ
Д№АИЃКЪЙШмвКГЪМюадЃЌвжжЦNaBO2 ЕФЫЎНт
ЂлЁАВйзї2ЁБЪЧНЋТЫвКеєЗЂЁЂНсОЇЁЂЯДЕгЃЌЕУЕНNaBO2ЃЌвђЮЊЦЋХ№ЫсФЦЃЈNaBO2ЃЉвзШмгкЫЎЃЌВЛШмгкввДМЃЌвзЫЎНтЃЌЫљвдЯДЕгбЁгУЕФЪдМСзюКУЪЧввДМЃЌЙЪбЁbЃЛ
Д№АИЃКb
ЂмЁАЗДгІ1ЁБЪЧMgH2гыNaBO2ЛьКЯЕУЕНNaBH4КЭMgOЃЌИљОнжЪСПЪиКуПЩЕУЃЌЛЏбЇЗНГЬЪНЮЊ2MgH2+NaBO2 =NaBH4+2MgOЃЛ
Д№АИЃК2MgH2+NaBO2 =NaBH4+2MgO
ЃЈ2ЃЉЕчНтЦЋХ№ЫсФЦЕФМюШмвКЃЌбєМЋЃК8OH--8e-=4H2O+2O2ЃЌвѕМЋЃКBO2-+8e-+6H2O=BH4-+8OH-ЃЛ
Д№АИЃКBO2-+8e-+6H2O=BH4-+8OH-



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПXЁЂYЁЂZЪЧЕкЂёAЁЋЂїAзхЕФШ§жжЗЧН№ЪєдЊЫиЃЌЫќУЧдкдЊЫижмЦкБэжаЕФЮЛжУШчЭМЫљЪОЃЌЪдЛиД№ЯТСаЮЪЬтЁЃ
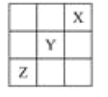
ЃЈ1ЃЉXдЊЫиЕЅжЪЕФЛЏбЇЪНЪЧ________ЁЃ
ЃЈ2ЃЉYдЊЫиЕФдзгНсЙЙЪОвтЭМЪЧ____________ЃЌYгыNaЫљаЮГЩЛЏКЯЮяЕФЕчзгЪНЮЊ________________________ЁЃ
ЃЈ3ЃЉZдЊЫиЕФУћГЦЪЧ________ЃЌДгдЊЫидзгЕУЪЇЕчзгЕФНЧЖШПДЃЌZдЊЫиОпга________адЃЛШєДгZдЊЫидкдЊЫижмЦкБэжаЫљДІЮЛжУПДЃЌЫќОпгаетжжаджЪЕФдвђЪЧ_________________________ЃЌЦфМлЕчзгХХВМЪНЮЊ__________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПBa(OH)2 ЪЧвЛжжЧПМюЃЌПЩгУгкВтЖЈЬьШЛЦјжа CO2 ЕФКЌСПЁЃ
ЃЈ1ЃЉCO2 ЕФУмЖШБШПеЦј______ЃЈЬюЁАДѓЁБЛђЁАаЁЁБЃЉ
ЃЈ2ЃЉЂйГЦШЁ5.25g Ъдбљ[КЌга Ba(OH)2xH2O КЭдгжЪ]ХфГЩ 100 mL ШмвКЃЌХфжЦШмвКжагУЕНЕФвЧЦїгаЬьЦНЁЂвЉГзЁЂСПЭВЁЂЩеБЁЂВЃСЇАєЁЂ______ЁЂНКЭЗЕЮЙмЁЃгУ 15.00 mL 0.2 mol/LбЮЫсгыЩЯЪі Ba(OH)2 ШмвКЗДгІЃЌЯћКФИУ Ba(OH)2ШмвК 10.00 mLЃЈдгжЪВЛгыЫсЗДгІЃЉ
ЂкСэШЁ5.25 g ЪдбљМгШШжСЪЇШЅШЋВПНсОЇЫЎЃЈдгжЪВЛЗжНтЃЉЃЌГЦЕУЪЃгрЙЬЬхжЪСПЮЊ3.09 gЃЌ Чѓ Ba(OH)2xH2OжаЕФ x=______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЬўЕФбмЩњЮяAЕФЛЏбЇЪНЮЊC6H12O2ЃЌвбжЊЃК

гжжЊDВЛгыNa2CO3ЗДгІЃЌCКЭEОљВЛФмЗЂЩњвјОЕЗДгІЃЌдђAЕФНсЙЙПЩФмгаЃЈЁЁЁЁЃЉ
A. 1жж B. 2жж C. 3жж D. 4жж
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГГЇЗЯЫЎжаКЌ5.00ЁС10-3molЁЄL-1ЕФ![]() ЃЌЦфЖОадНЯДѓЁЃФГбаОПадбЇЯАаЁзщЮЊСЫБфЗЯЮЊБІЃЌНЋЗЯЫЎДІРэЕУЕНДХадВФСЯ
ЃЌЦфЖОадНЯДѓЁЃФГбаОПадбЇЯАаЁзщЮЊСЫБфЗЯЮЊБІЃЌНЋЗЯЫЎДІРэЕУЕНДХадВФСЯ![]() ЃЈ
ЃЈ![]() ЕФЛЏКЯМлвРДЮЮЊ+3ЁЂ+2ЃЉЃЌЩшМЦСЫШчЯТЪЕбщСїГЬЃК
ЕФЛЏКЯМлвРДЮЮЊ+3ЁЂ+2ЃЉЃЌЩшМЦСЫШчЯТЪЕбщСїГЬЃК
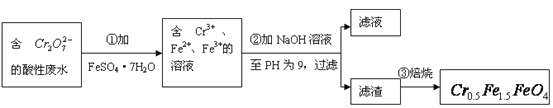
ЃЈ1ЃЉЕкЂйВНЗДгІЕФРызгЗНГЬЪНЪЧ_________________________________________________ЃЛ
ЃЈ2ЃЉЕкЂкВНжагУpHЪджНВтЖЈШмвКpHЕФВйзїЪЧЃК
______________________________________________________________________________ЃЛ
ЃЈ3ЃЉЕкЂкВНЙ§ТЫЕУЕНЕФТЫдќжажївЊГЩЗжГ§CrЃЈOHЃЉ3ЭтЃЌЛЙга______________________ЃЛ
ЃЈ4ЃЉгћЪЙ1LИУЗЯЫЎжаЕФ![]() ЭъШЋзЊЛЏЮЊ
ЭъШЋзЊЛЏЮЊ![]() ЁЃРэТлЩЯашвЊМгШы__________g FeSO4ЁЄ7H2OЁЃ
ЁЃРэТлЩЯашвЊМгШы__________g FeSO4ЁЄ7H2OЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊгааЇПижЦЮэіВЃЌИїЕиЛ§МЋВЩШЁДыЪЉИФЩЦДѓЦјжЪСПЃЌбаОПВЂгааЇПижЦПеЦјжаЕФЕЊбѕЛЏЮяЁЂЬМбѕЛЏЮяКЌСПЯдЕУгШЮЊживЊЁЃ
ЂёЃЎЕЊбѕЛЏЮябаОП
ЃЈ1ЃЉвЛЖЈЬѕМўЯТЃЌНЋ2molNOгы2molO2жУгкКуШнУмБеШнЦїжаЗЂЩњЗДгІ2NO(g)+O2(g)![]() 2NO2(g)ЃЌЯТСаИїЯюФмЫЕУїЗДгІДяЕНЦНКтзДЬЌЕФЪЧ____________ЁЃ
2NO2(g)ЃЌЯТСаИїЯюФмЫЕУїЗДгІДяЕНЦНКтзДЬЌЕФЪЧ____________ЁЃ
aЃЎЬхЯЕбЙЧПБЃГжВЛБф bЃЎЛьКЯЦјЬхУмЖШБЃГжВЛБф
cЃЎNOКЭO2ЕФЮяжЪЕФСПжЎБШБЃГжВЛБф dЃЎУПЯћКФ2molNOЭЌЪБЩњГЩ2 molNO2
ЃЈ2ЃЉЦћГЕФкШМЛњЙЄзїЪБЛсв§Ц№N2КЭO2ЕФЗДгІЃКN2+ O2![]() 2NOЃЌЪЧЕМжТЦћГЕЮВЦјжаКЌгаNOЕФдвђжЎвЛЁЃдкT1ЁЂT2ЮТЖШЯТЃЌвЛЖЈСПЕФNOЗЂЩњЗжНтЗДгІЪБN2ЕФЬхЛ§ЗжЪ§ЫцЪБМфБфЛЏШчгвЭМЫљЪОЃЌИљОнЭМЯёХаЖЯЗДгІN2ЃЈgЃЉ+ O2ЃЈgЃЉ
2NOЃЌЪЧЕМжТЦћГЕЮВЦјжаКЌгаNOЕФдвђжЎвЛЁЃдкT1ЁЂT2ЮТЖШЯТЃЌвЛЖЈСПЕФNOЗЂЩњЗжНтЗДгІЪБN2ЕФЬхЛ§ЗжЪ§ЫцЪБМфБфЛЏШчгвЭМЫљЪОЃЌИљОнЭМЯёХаЖЯЗДгІN2ЃЈgЃЉ+ O2ЃЈgЃЉ![]() 2NOЃЈgЃЉЕФЁїH____0(ЬюЁАЃОЁБЛђЁАЃМЁБ)ЁЃ
2NOЃЈgЃЉЕФЁїH____0(ЬюЁАЃОЁБЛђЁАЃМЁБ)ЁЃ

ЃЈ3ЃЉNOxЪЧЦћГЕЮВЦјжаЕФжївЊЮлШОЮяжЎвЛЁЃЦћГЕЗЂЖЏЛњЙЄзїЪБЛсв§ЗЂN2КЭO2ЗДгІЃЌ
ЦфФмСПБфЛЏШчЭМЫљЪОЃК
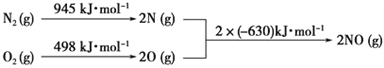
аДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪНЃК________________________________ЁЃ
ЂђЃЎЖўбѕЛЏСђКЭЕЊЕФбѕЛЏЮяЪЧГЃгУЕФЛЏЙЄдСЯЃЌЕЋвВЪЧДѓЦјЕФжївЊЮлШОЮяЁЃзлКЯжЮРэЦфЮлШОЪЧЛЕОГЛЏбЇЕБЧАЕФживЊбаОПФкШнжЎвЛЁЃ
ЃЈ1ЃЉСђЫсЩњВњжаЃЌSO2ДпЛЏбѕЛЏЩњГЩSO3ЃК2SO2(s)+O2ЃЈgЃЉ![]() 2SO3ЃЈgЃЉЃЌФГЮТЖШЯТЃЌSO2ЕФЦНКтзЊЛЏТЪ(ІС)гыЬхЯЕзмбЙЧП(P)ЕФЙиЯЕШчЯТЭМЫљЪОЁЃИљОнЭМЪОЛиД№ЯТСаЮЪЬтЃК
2SO3ЃЈgЃЉЃЌФГЮТЖШЯТЃЌSO2ЕФЦНКтзЊЛЏТЪ(ІС)гыЬхЯЕзмбЙЧП(P)ЕФЙиЯЕШчЯТЭМЫљЪОЁЃИљОнЭМЪОЛиД№ЯТСаЮЪЬтЃК
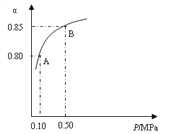
ЂйНЋ2.0 molSO2КЭ1.0molO2жУгк10 LУмБеШнЦїжаЃЌЗДгІДяЦНКтКѓЃЌЬхЯЕзмбЙЧПЮЊ0.10MPaЁЃИУЗДгІЕФЦНКтГЃЪ§ЕШгк__________ЁЃ
ЂкЦНКтзДЬЌгЩAБфЕНBЪБЃЌЦНКтГЃЪ§KЃЈAЃЉ_______K(B)(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁА=ЁБ)ЁЃ
ЃЈ2ЃЉгУCH4ДпЛЏЛЙдNOxПЩвдЯћГ§ЕЊбѕЛЏЮяЕФЮлШОЁЃР§ШчЃК
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ЁїH=-574kJЁЄmol-1
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ЁїH=-1160kJЁЄmol-1
ШєгУБъзМзДПіЯТ4.48 L CH4ЛЙдNO2жСN2ЃЌећИіЙ§ГЬжазЊвЦЕФЕчзгзмЪ§ЮЊ__________(АЂЗќМгЕТТоГЃЪ§ЕФжЕгУNAБэЪО)ЃЌЗХГіЕФШШСПЮЊ___________kJЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЛЏбЇаЫШЄаЁзщЖдЕчНтжЪШмвКзїШчЯТЕФЙщФЩзмНсЃЈОљдкГЃЮТЯТЃЉЁЃЦфжае§ШЗЕФЪЧ
A. pH=3ЕФЧПЫсШмвК1mLЃЌМгЫЎЯЁЪЭжС100mLКѓЃЌШмвКpHНЕЕЭ2ИіЕЅЮЛ
B. 1L 0.50molЁЄL-1NH4Cl ШмвКгы2L 0.25molЁЄL-1NH4Cl ШмвККЌNH4+ЮяжЪЕФСПКѓепДѓ
C. pH=8.3ЕФNaHCO3ШмвКЃКc(Na+)ЃОc(HCO3Ѓ)ЃОc(CO32-)ЃОc(H2CO3)
D. pH=4ЁЂХЈЖШОљЮЊ0.1molЁЄL-1ЕФCH3COOHЁЂCH3COONaЛьКЯШмвКжаЃКc(CH3COOЃ)Ѓc(CH3COOH)=2[c(HЃЋ)Ѓc(OHЃ)]=![]()
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩшNAЮЊАЂЗќйЄЕТТоГЃЪ§ЕФжЕЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. 2L 0.5mol/LбЧСђЫсШмвКжаКЌгаЕФH+РызгЪ§ЮЊ2NA
B. ЪвЮТЯТЃЌ1L pH=13ЕФNaOHШмвКжаЃЌгЩЫЎЕчРыЕФOH-РызгЪ§ФПЮЊ0.1NA
C. ЕчНтОЋСЖЭЕФЙ§ГЬжаЃЌУПзЊвЦNAИіЕчзгЪБЃЌбєМЋШмНтЭЕФжЪСПЮЊ32g
D. ФГУмБеШнЦїжаЪЂга0.1mol N2КЭ0.3mol H2ЃЌдквЛЖЈЬѕМўЯТГфЗжЗДгІЃЌзЊвЦЕчзгЕФЪ§ФПаЁгк0.6NA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗфНКЪЧвЛжжЬьШЛПЙАЉвЉЃЌжївЊЛюадГЩЗжЮЊПЇЗШЫсБНввѕЅ(J)ЁЃКЯГЩЛЏКЯЮяIЕФТЗЯпШчЯТЃК
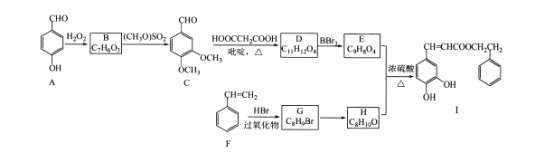
вбжЊЃКЂй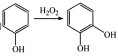
ЂкRCHO+HOOCCH2COOH![]() RCH=CHCOOH
RCH=CHCOOH
ЂлЕБєЧЛљгыЫЋМќЬМдзгЯрСЌЪБЃЌвзЗЂЩњзЊЛЏЃКRCH-CHOH=RCHCHO
ЧыЛиД№ЯТСаЮЪЬтЃК
(l)ЛЏКЯЮяFЕФУћГЦЪЧ____;B-CЕФЗДгІРраЭЪЧ____ЁЃ
(2)ЛЏКЯЮяEжаКЌбѕЙйФмЭХЕФУћГЦЪЧ____ЃЛG-HЕФЗДгІЫљашЪдМСКЭЬѕМўЗжБ№ЪЧ____ЁЂ____ЁЃ
(3)аДГіЛЏКЯЮяCгыаТжЦCu(OH)2аќзЧвКЗДгІЕФЛЏбЇЗНГЬЪН____ЁЃ
(4)ЛЏКЯЮяWгыEЛЅЮЊЭЌЗжвьЙЙЬхЃЌСНепЫљКЌЙйФмЭХжжРрКЭЪ§ФПЭъШЋЯрЭЌЃЌЧвБНЛЗЩЯга3ИіШЁДњЛљЃЌдђWПЩФмЕФНсЙЙга____жж(ВЛПМТЧЫГЗДвьЙЙ)ЃЌЦфжаКЫДХЙВеёЧтЦзЯдЪОга6жжВЛЭЌЛЏбЇЛЗОГЕФЧтЃЌЗхУцЛ§БШЮЊ2:2:1ЃК1:1:1ЃЌаДГіЗћКЯвЊЧѓЕФWЕФНсЙЙМђЪН____ЁЃ
(5)ВЮееЩЯЪіКЯГЩТЗЯпЃЌЩшМЦгЩCH3CH=CH2КЭHOOCCH2COOHЮЊдСЯжЦБИ CH3CH2CH=CHCOOHЕФКЯГЩТЗЯп(ЮоЛњЪдМСШЮбЁ)____ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com