
����Ŀ���Թ�ҵ��ˮ��������ˮ���д����Ƿ�ֹˮ����Ⱦ������ˮ�ʵ���Ҫ��ʩ֮һ�����᳧�����Է�ˮ����(As)Ԫ��(��Ҫ��H3AsO3��ʽ����)�������ߣ�Ϊ��������ŷţ�ij�������û�ѧ���������������ˮ����ش��������⣺
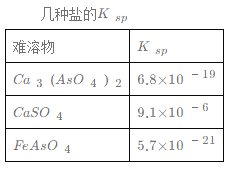
��1�������Է�ˮ��Fe3+��Ũ��Ϊ1.0��10-4 mol��L-1����c(AsO43-)������ ____mol��L-1��
��2�������ŷų������Է�ˮ�е�������(����H3AsO3)���׳�������Ͷ��MnO2�Ƚ�������������� (����H3AsO4)����ʱMnO2����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ_________________��
��3������(H3AsO4)�ֲ������ƽ�ⳣ��(25 ��)ΪKa1=5.6��10-3��Ka2=1.7��10-7��Ka3=4.0��10-12�������������ƽ�ⳣ������ʽΪKa3=_________��Na3AsO4��һ��ˮ������ӷ���ʽΪAsO43��+H2O![]() HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��(25 ��)Ϊ____��
HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��(25 ��)Ϊ____��
���𰸡�5.7��10-17 2H++MnO2+H3AsO3![]() H3AsO4+Mn2++H2O
H3AsO4+Mn2++H2O ![]() 2.5��10-3
2.5��10-3
��������
������Ҫ�������ܵ���ʵ��ܽ�ƽ�⼰����ת���ı��ʡ�
��1�������ܶȻ���������õ�AsO43-��Ũ�ȣ�
��2�����ݷ�Ӧ����������ϵ�����Ԫ�ػ��ϼ۵ı仯��ƽ����ʽ��
��3��H3AsO4�ĵ���������ʽΪHAsO42-![]() H++AsO43-������д�������ƽ�ⳣ���ı���ʽ������ˮ�ⳣ������볣����Kw�Ĺ�ϵ������
H++AsO43-������д�������ƽ�ⳣ���ı���ʽ������ˮ�ⳣ������볣����Kw�Ĺ�ϵ������
��1������Ksp(FeAsO4)=c(Fe3+)��c(AsO43-)=5.7��1021��Fe3+��Ũ��Ϊ1.0��104molL1����c(AsO43-)=Ksp(FeAsO4)/c(Fe3+)=5.7��1017mol/L��
��2��������(H3AsO3����)���׳�������Ͷ��MnO2�Ƚ��������������(H3AsO4����)����÷�Ӧ�����ӷ���ʽΪ��2H++MnO2+H3AsO3===H3AsO4+Mn2++H2O��
��3��H3AsO4�ĵ���������ʽΪHAsO42��![]() H++ AsO43�������Ե����������ƽ�ⳣ���ı���ʽΪ
H++ AsO43�������Ե����������ƽ�ⳣ���ı���ʽΪ![]() ��Na3AsO4�ĵ�һ��ˮ������ӷ���ʽΪ��AsO43��+H2O
��Na3AsO4�ĵ�һ��ˮ������ӷ���ʽΪ��AsO43��+H2O![]() HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��
HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��![]() ��
��


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��BaTiO3��KH2PO4��NaNO2�����������壬���Ǿ�����������������統����ѹ�����ı���״ʱ����������ͨ��ʱ��ı���״�ȡ�
(1)��̬Tiԭ�ӵļ۵����Ų�ʽΪ___________������___________��Ԫ�ء�
(2)KH2PO4�����д��ڵ���������___________(ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.���
(3)NO2-��Nԭ�ӵ��ӻ�����Ϊ___________������(����>��<��=��)___________120������ԭ����___________��N��O��Na����Ԫ�صĵڶ�������(I2)�ɴ�С��˳��Ϊ___________(��Ԫ�ط��ű�ʾ)��
(4)BaTiO3�ľ�������ͼ��ʾ��

Tiԭ�ӵ���λ��Ϊ___________��������ܶ�Ϊ��g/cm3�������Baԭ�Ӻ�Oԭ��֮��ľ���Ϊ___________�������ʽ)nm��( BaTiO3��Ħ������Ϊ233g/mol��NAΪ�����ӵ�������ֵ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ȼˮ��ຬCa2+��Mg2+��HCO3-�����ӣ����Ȼ����ˮ����ˮ����һ������CaCO3��Mg(OH)2�����ܺ���MgCO3��
(1)��Ȼˮ�е�HCO3-�����ڿ����е�CO2������ط���ʽ��ʾCO2����ˮ�γ�HCO3-�Ĺ��̡�______________________________________________________________
(2)��Ȼˮ���ʱ�������ܵ�MgCO3ת�������ܵ�Mg(OH)2��д��������Ӧ�Ļ�ѧ����ʽ��____________________________________________
Ϊȷ��ijˮ����Ʒ�ijɷ֣�����CaCO3��MgCO3��Mg(OH)2���·ֽ�����ʣ���ȷ����5.000gˮ����Ʒ��������ͼװ�ý���ʵ�顣
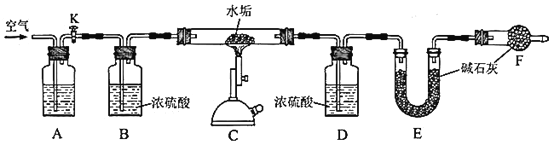
(3)A��ʢ�ŵ��Լ���__________��װ��F��������_________________________����Ӧ��������Ҫ��ͨ��һ��ʱ��Ŀ�����Ŀ����_______________________��
(4)��������װ�òⶨˮ����Mg(OH)2�ĺ���ʱ����Ҫ������������__________��
(5)ʵ����װ��E����2.200g������ˮ����Ʒ���Ƿ���MgCO3?�ж�������_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƺ��¹���������Ļ��Һ����Ϊ̼�ظֵĻ�ʴ���������£�̼�ظ������ֲ�ͬ�����еĸ�ʴ����ʵ������ͼ��ʾ������˵������ȷ����
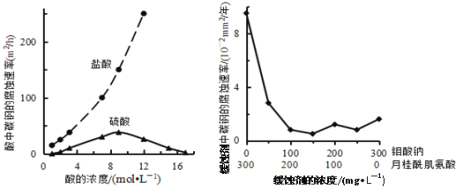
A. �����Ũ��Խ��ʴ����Խ��
B. �����ƺ��¹����������Ũ�����ʱ����ʴ������С
C. ̼�ظֵĸ�ʴ���ʲ��������Ũ�����������˵����Ӧ���ʲ���c(H+)������
D. �Ա�����������������ߣ���֪Cl��Ҳ��Ӱ��̼�ظֵĸ�ʴ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�Ǿ����Դ���⣬��ˮ���������ۺ����þ�����Ҫ���塣

��ش��������⣺
��1������I�У������к���Ca2+��Mg2+��SO42-���������ӣ����ξ��ƹ�����Ҫʹ��Na2CO3��Һ����д������Na2CO3��Һ����ػ�ѧ��Ӧ�����ӷ���ʽ��_________________________��
��2����ˮ���壬�Ƶ�1molBr2������Ҫ����_________molCl2�����������Na2SO3ˮ��Һ����Br2���йط�Ӧ�����ӷ���ʽΪ_________��
��3�������Ȼ�̼���Խ����ɵ�����ȡ���������������________���ʣ�Ϊ�˳�ȥ�����в���������Cl2���������м���_________��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������⣩
(һ)��������ѧ���о�����������ϩ������Ϊԭ�ϡ��Ӷ����������ϳ������������¹��գ����������Ҵ�����ȩ���м��壬ʹ��Ʒ�ɱ����ͣ��������Ծ������ơ���ϳɵĻ�����Ӧ���£�
CH2=CH2(g)+CH3COOH(1)![]() CH3COOC2H5(1)
CH3COOC2H5(1)
(1)����������˵���̶���������ϩ������ϳ����������ķ�Ӧ�Ѵﻯѧƽ�����___________��
A�����ᡢ����������Ũ����ͬ
B�������ϳɷ�Ӧ�����������ֽⷴӦ���������
C����ϵ�������ܶ�һ��
D����ϩ�Ͽ�1mol̼̼˫����ͬʱ����ǡ������1mol
(2)��n(��ϩ)��n(����)���ϱ�Ϊ1�������£�ij�о�С���ڲ�ͬѹǿ�½���������ͬʱ������������IJ������¶ȵı仯�IJⶨʵ�飬ʵ������ͼ��ʾ���ش��������⣺
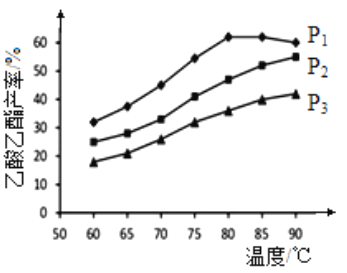
���¶���60��90�淶Χ�ڣ���ϩ�����������ϳɷ�Ӧ�����ɴ�С��˳����___________[��v(P1)��v(P2)��v(P3)�ֱ��ʾ��ͬѹǿ�µķ�Ӧ����]��������ԭ��Ϊ______________________��
����ѹǿΪP1MPa���¶ȳ���80��ʱ���������������½���ԭ�������______________________��
�۸��ݲⶨʵ���������������˵�����������______________________(������ʵ�ѹǿ���¶�)��Ϊ������������IJ����ʹ��ȣ����Բ�ȡ�Ĵ�ʩ��______________________(��д��һ��)��
(3)��֪��Ӧ�۵ı�ƽ�ⳣ��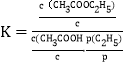 ������c��Ϊ��Ũ��(1.0mol/L)��p(C2H4)Ϊƽ��ϵͳ��C2H4��ƽ���ѹ��p(C2H4)=p��x(C2H4)��x(C2H4)Ϊƽ��ϵͳ��C2H4�����������p��Ϊ��ѹǿ(1.0��105Pa)���������ʵ�������ϩ��������80���10MPa�·�Ӧ������������ƽ�����Ϊ80%����K��=__________��
������c��Ϊ��Ũ��(1.0mol/L)��p(C2H4)Ϊƽ��ϵͳ��C2H4��ƽ���ѹ��p(C2H4)=p��x(C2H4)��x(C2H4)Ϊƽ��ϵͳ��C2H4�����������p��Ϊ��ѹǿ(1.0��105Pa)���������ʵ�������ϩ��������80���10MPa�·�Ӧ������������ƽ�����Ϊ80%����K��=__________��
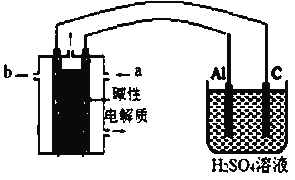
(��)ij��ѧ��ȤС����ʵ������ģ������Ʒ�������ۻ�����������(װ����ͼ��ʾ)���ɹ�ѡ����Լ��У��״���������KOH��Һ����������b��__________�����缫�ĵ缫��ӦΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������һƿ��84����Һ������װ˵�����£����з�������ȷ���ǣ� ��

A.��84����Һ������ʱ���ܳ��ڷ��ã���Ҫ�ܷⱣ��
B.����84����Һ����NaClO�����ʵ���Ũ��ԼΪ4mol��L-1
C.ȡ100mL����84����Һ��ϡ��100��������������ϡ�ͺ����Һ��c��Na+��ԼΪ0.04mol��L-1
D.����NaClO�������ƺ�25% NaClO������Һ480mL����Ҫ������NaClO��������Ϊ142.8g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС��ͬѧ̽�����ʵ��ܽ�ȴ�С�����ת������֮��Ĺ�ϵ����֪��
���� | BaSO4 | BaCO3 | AgI | AgCl | |
�ܽ��/g��20�棩 | 2.4��10-4 | 1.4��10-3 | 3.0��10-7 | 1.5��10-4 |
��1��̽��BaCO3��BaSO4֮���ת��
ʵ�������
�Լ�A | �Լ�B | �Լ�C | �������������� | |
ʵ��� | BaCl2 | Na2CO3 | Na2SO4 | ���� |
ʵ��� | Na2SO4 | Na2CO3 | ���������ݲ��������������ܽ� |
�� ʵ���˵��BaCO3ȫ��ת��ΪBaSO4�����ݵ������Ǽ��������______��
�� ʵ����м���ϡ���������Ӧ�����ӷ���ʽ��______��
�� ʵ���˵�����������˲���ת�������BaSO4�ij����ܽ�ƽ�����ԭ��______��
��2��̽��AgCl��AgI֮���ת��
ʵ���
ʵ��������Թ��н�����Һ�䷴Ӧʱ��ͬѧ�����۲쵽AgIת��ΪAgCl�����������������ʵ�飨��ѹ��������a��c��b��0����
װ�� | ���� | ��ѹ������ | |
| ��.��ͼ����װ�ò������Լ����պ�K | a | |
��.��B�е���AgNO3(aq)����������ȫ | b | ||
��.����B��Ͷ��һ����NaCl (s) | c | ||
��.�ظ���������B�м����뢣����NaCl(s) | a |
ע��������������ʱ������ԭ��ط�Ӧ������������ԭ�����������ԣ���ԭ�ԣ�Խǿ��ԭ��صĵ�ѹԽ�����ӵ������ԣ���ԭ�ԣ�ǿ������Ũ���йء�
�� ʵ���֤����AgClת��ΪAgI������Һ������______������ţ���
a. AgNO3��Һ b. NaCl��Һ c. KI��Һ
�� ʵ����IJ��袡�У�B��ʯī�ϵĵ缫��Ӧʽ��______��
�� �����Ϣ������ʵ�����b��a��ԭ��______��
�� ʵ�����������˵��AgIת��ΪAgCl��������______��
��3���ۺ�ʵ������ɵó����ۣ� ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A. CHCl3��HF �� CHFCl2��HCl ����ȡ����Ӧ
B. ú�������� CO ��H2���پ������ϳɿ��Եõ��״���Һ��ȼ��
C. ʯ���ѽ�����SO2 ����ʹ KMnO4 ��Һ��ɫ����ɫԭ����ͬ
D. ����һ������������H2 �����ӳɷ�Ӧ��Ҳ�������������ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com