
| �ټ�����ˮ |
| �ܽ⣬���� |
| �ڼӹ���x�Լ� |
| ��Mg2+ |
| �ۼӹ���y�Լ� |
| ��A���� |
| �ܼӹ���z�Լ� |
| ��()���� |
| �� |
| ������ |
| ���������� |
| ��()���� |
| �� |
| �����ᾧ |


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮
�ס�������ʵ��С��ֱ���С��ⶨNa2CO3��NaCl�������Na2CO3��������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
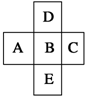 ��Ԫ�����ڱ��е�ǰ�����ڣ��������ڵ�5��Ԫ����ͼ��ʾ����BԪ�صĺ˵����Ϊa������˵����ȷ���ǣ�������
��Ԫ�����ڱ��е�ǰ�����ڣ��������ڵ�5��Ԫ����ͼ��ʾ����BԪ�صĺ˵����Ϊa������˵����ȷ���ǣ�������| A��B��D��ԭ������֮�����Ϊ2 |
| B��E��B��ԭ������֮�������8��18��32 |
| C��5��Ԫ�صĺ˵������֮�Ϳ���Ϊ5a+10 |
| D��A��E��ԭ������֮�������7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 3 |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | A | B | C | D |
| ���ʽṹ��Ϣ | ԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��� | ԭ�ӵ�M����1�ԳɶԵ�p���� | ԭ�Ӻ�������Ų�Ϊ[Ar]3d104sx����+1��+2���ֳ������ϼ� | �����ֳ��������������һ����ұ��ҵ���õĻ�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ش��� | I1 | I2 | I3 | I4 |
| E | 740 | 1500 | 7700 | 10500 |
| F | 580 | 1800 | 2700 | 11600 |
| G | 420 | 3100 | 4400 | 5900 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��10.7g |
| B��21.4g |
| C��23.9g |
| D��40.0g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | ���볣����Ka�� | �� | ���볣����Ka�� |
| CH3COOH | 1.8��10-5 | H3PO4 | K1=7.1��10-3 |
| H3PO3 | K1=3.7��10-2 | K2=6.2��10-8 | |
| K2 =2.9��10-7 | K3=4.5��10-13 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com