
����Ŀ���ܻ���Ӧ��һ��ͬʱ�ɼ���ϼ��ķ�Ӧ��������ܻ���Ӧ��Diels-Alder��Ӧ![]() ���绷����Ӧ(electrocyclic reaction)��
���绷����Ӧ(electrocyclic reaction)��![]() �ȡ�
�ȡ�
��֪��![]() ������R1��R2��R3Ϊ�����
������R1��R2��R3�����
����һ����Ԫ��״����I�ĺϳ�·������(A~I����ʾһ���л�������)��
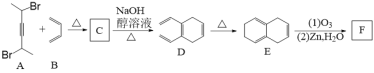
![]()
�ش��������⣺
��1��������A�к��еĹ�����������_____________��D�ķ���ʽΪ________
��2����C����D�ķ�Ӧ����Ϊ_____________��A�γɸ߾���Ľṹ��ʽΪ__________________
��3����F����G�Ļ�ѧ����ʽΪ____________________________________________
��4��������H��ϵͳ����Ϊ_________________
��5��������I�����ױ�������Է�������Ϊ142��I�ĺ˴Ź���������ʾΪ2��壬I�Ľṹ��ʽΪ________________________
��6���ڻ�����I��ͬ���칹������ͬʱ����������������____________(��д�ṹ��ʽ)
�� ���зӽṹ���� ����һ�ֹ����ţ��� ����4�ֵ�Ч��
���𰸡�̼̼��������ԭ��C10H12��ȥ��Ӧ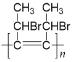
![]() + 4Ag(NH3)2OH
+ 4Ag(NH3)2OH ![]()
![]() +2H2O+4Ag��+6NH33,4-���ǻ�������
+2H2O+4Ag��+6NH33,4-���ǻ�������![]()
��������
��������ͼ��AΪ![]() ����B��
����B��![]() �������ܻ���Ӧ����C��CΪ
�������ܻ���Ӧ����C��CΪ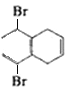 ��C���������ƴ���Һ�з�����ȥ��Ӧ����D��DΪ
��C���������ƴ���Һ�з�����ȥ��Ӧ����D��DΪ![]() ��D�ڼ���ʱ�����绷����Ӧ����E������
��D�ڼ���ʱ�����绷����Ӧ����E������![]() ��E��̼̼˫���ڳ������������·�Ӧ����F��FΪ
��E��̼̼˫���ڳ������������·�Ӧ����F��FΪ![]() ��
��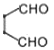 ���������I����������Ԫ������֪FΪ
���������I����������Ԫ������֪FΪ![]() ��F����������Ӧ������������G��GΪ
��F����������Ӧ������������G��GΪ![]() ��G�������ӳ�����H��HΪ
��G�������ӳ�����H��HΪ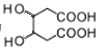 ��H��Ũ������������������I����������Ԫ������������I�����ױ�������Է�������Ϊ142��I�ĺ˴Ź���������ʾΪ2��壬��IΪ
��H��Ũ������������������I����������Ԫ������������I�����ױ�������Է�������Ϊ142��I�ĺ˴Ź���������ʾΪ2��壬��IΪ![]() ����1��������AΪ
����1��������AΪ![]() �����к��еĹ�����������̼̼��������ԭ�ӣ�DΪ
�����к��еĹ�����������̼̼��������ԭ�ӣ�DΪ![]() ������ʽΪC10H12����2�����ݷ�Ӧ��������C����D��±��������ȥ��Ӧ���ʷ�Ӧ����Ϊ��ȥ��Ӧ��AΪ
������ʽΪC10H12����2�����ݷ�Ӧ��������C����D��±��������ȥ��Ӧ���ʷ�Ӧ����Ϊ��ȥ��Ӧ��AΪ![]() ��ͨ���Ӿ۷�Ӧ�γɸ߾���Ľṹ��ʽΪ
��ͨ���Ӿ۷�Ӧ�γɸ߾���Ľṹ��ʽΪ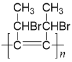 ����3��FΪ
����3��FΪ![]() ��
��![]() ������������Һ��Ӧ���ữ����G������F����G�Ļ�ѧ����ʽΪ
������������Һ��Ӧ���ữ����G������F����G�Ļ�ѧ����ʽΪ![]() + 4Ag(NH3)2OH
+ 4Ag(NH3)2OH ![]()
![]() +2H2O+4Ag��+6NH3����4��������HΪ
+2H2O+4Ag��+6NH3����4��������HΪ ��ϵͳ����Ϊ3,4-���ǻ�����������5����������������������I�Ľṹ��ʽΪ
��ϵͳ����Ϊ3,4-���ǻ�����������5����������������������I�Ľṹ��ʽΪ![]() ����6���� ���зӽṹ��˵�����з��ǻ����� ����һ�ֹ����ţ�˵��ֻ���з��ǻ���ͬʱ���б������� ����4�ֵ�Ч�⣬����������ͬ���칹����
����6���� ���зӽṹ��˵�����з��ǻ����� ����һ�ֹ����ţ�˵��ֻ���з��ǻ���ͬʱ���б������� ����4�ֵ�Ч�⣬����������ͬ���칹����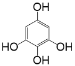 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����c(CH3COOH)+ c(CH3COO��)=0.1mol/L�Ĵ��ᡢ�����ƻ����Һ��c(CH3COOH)��c(CH3COO��) ��pH�Ĺ�ϵ��ͼ��ʾ�������й���Һ����������ȷ���ǡ�
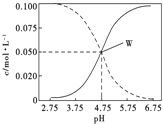
A. pH=5.5����Һ��c(CH3COOH)��c(CH3COO��)��c(H+)��c(OH��)
B. ��Һ�У�c(H+)+ c(Na+)=c(CH3COO��)+c(OH��)
C. ��W��������25��ʱCH3COOH�ĵ��볣��
D. pH=4����Һ��: c(H+)+ c(Na+)+c(CH3COOH)=0.1mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����I��һ�ֺϳ�·�����£�

�ش��������⣺
(1)C������Ϊ______________;H����������������______________��
(2)B��C�ķ�Ӧ������______________;д��I�Ľṹ��ʽ��______________��
(3)G���������______________��ԭ�ӹ�ƽ�档
(4)д��A��B�Ļ�ѧ����ʽ��____________________________��
(5)J��I��ͬ���칹��,ͬʱ��������������J�Ľṹ��_______�֡����У�һ�ֺ˴Ź���������6�����ҷ�����֮��Ϊ1��1��1��2��2��3�Ľṹ��ʽΪ______________��
����ʹ������Ȼ�̼��Һ��ɫ
������̼��������Һ��Ӧ����CO2
�����ڷ����廯����ұ����ϵ�һ�ȴ���ֻ��2��
(6)������������,����ϩ��OHC-CHOΪԭ�Ϻϳ�HOOCCH=CHCOOH,��ƺϳ�·�ߣ�___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����6.2 g�л���A������O2���ܱ������г��ȼ�գ�����ֻ��H2O��CO2������ͨ��ŨH2SO4����������5.4g����ͨ����ʯ����ȫ���գ���������8.8g�����������A�ķ�����Ϊ62;
��1��д�������ʽ_____________________��
��2����0.2mol�ĸ��л���ǡ����9.2g�Ľ�������ȫ��Ӧ�����㲢�ƶϳ����л���Ľṹ��ʽ____________��
��3�����ں˴Ź��������н������___________���ź�.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ڹ�ҵ������Ҫ��;����ͭ������Ǧ�ȵ��յ�����������������Ҫ��������ڻ��P����״̬���ڡ�һ�ִ�����������ȡSe��Te�Ĺ������̼��£�
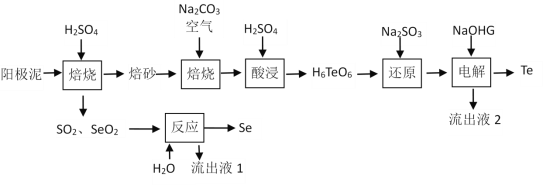
��֪��������(Na2H4TeO6)���ܣ�����(H6TeO6)���ܡ�
�ش��������⣺
��1����������600 K���Ҽ���һ��Ũ��H2SO4��������ʱ������Teת��ΪTeO2�Ļ�ѧ����ʽΪ___________________________________________��
��2�� ����ɰ����̼���Ƴ�ֻ�ϣ������ͨ�����������ϣ�ÿ����1 mol�����ƣ�����Ҫ��������O2����Ϊ_______mol�����ɵ������Ʋ�ˮ��������ȡ���������ԭ����______________________________________��
��3�� ����Ӧ��ʱ�Ļ�ѧ����ʽΪ_________________________________________________��
��4������ԭ���еķ�Ӧ������ΪTeO2�����鷴Ӧ���Ƿ��з�Ӧ��Na2SO3������ʵ���������Ϊ________________________________________________________��
��5����֪�������ʱʹ��ʯī�缫������������������������ʵ���֮��Ϊ______________��
��6������·���п���ѭ�����õ�������_____________________��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ǽ������ϵ����ǣ���������������Լռ�ؿ�������90%���ϡ�
(1)SiO2�Dz����ijɷ�֮һ��SiO2������������Һ��Ӧ�Ļ�ѧ����ʽΪ______________________________������ʦ����________(����������)����̲�����
(2)��Na2SiO3��Һ���ݹ���������ȼ�գ�����Na2SiO3����;����________��ԭ�ϡ�
(3)��ҵ�ϳ���2C��SiO2![]() Si��2CO���Ʊ��赥�ʣ��÷�Ӧ����Ԫ�ػ��ϼ����ߵ�������________(�ѧʽ����ͬ)����������________��
Si��2CO���Ʊ��赥�ʣ��÷�Ӧ����Ԫ�ػ��ϼ����ߵ�������________(�ѧʽ����ͬ)����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ӧ��ϩ����������õ�����һ������A������ʽΪC9H20����ȴ�������κ�C9H18��ϩ��������õ���������A������ͬ���칹��B1��B2��B3���ֱ���Զ���ֻ����һ����Ӧ��ϩ��������õ����ƶϲ�д��A��B1��B2��B3�Ľṹ��ʽΪ��A________��B1______________��B2__________��B3_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2SO2(g)+ O2(g) ![]() 2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж���ȷ����
2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж���ȷ����
�� | �� | �� | ||
��ʼ���ʵ��� | n(SO2)/mol | 0.4 | 0.8 | 0.8 |
n(O2)/mol | 0.24 | 0.24 | 0.48 | |
SO2��ƽ��ת����/% | 80 | ��1 | ��2 |
A. ���з�Ӧ��ƽ�ⳣ��С����
B. ���¶��£�ƽ�ⳣ��ֵΪ400
C. ƽ��ʱ������c(SO3)�Ǽ��е�2��
D. ƽ��ʱ������O2��ת���ʴ�������O2��ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ���ҳ��õIJ�����������ش��������⣺
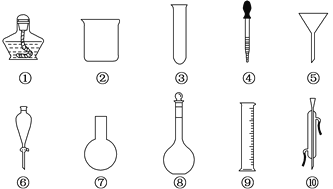
��1�����Ϊ��͢�����������Ʒֱ�Ϊ____________________��_________________��
��2���ڷ�Һ�����У������õ����������е�____________(�����)��
��3��������Ӧ�����ҿ�ֱ�Ӽ��ȵ������������е�____________(������)��
��4������һ�����ʵ���Ũ�ȵ���ҺʱҪ�õ���������____________(�����)��
��5�������ϱ����¶ȵ���____________(�����)��
��6����ͼ���¶ȼơ���Ͳ���ζ��ܵ�һ���֣���������(���߶�Ӧ�̶�)��˵����ȷ����________��
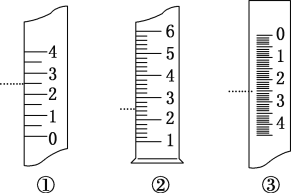
A��������Ͳ������Ϊ2.5 mL
B��������Ͳ������Ϊ2.5 mL
C�����ǵζ��ܣ�����Ϊ2.5 mL
D�������¶ȼƣ�����Ϊ2.5 ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com