
| 57 |
| 12 |
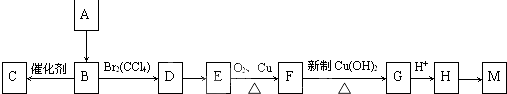
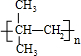 ����CH3��2C=CH2���巢���ӳɷ�Ӧ����DΪ��CH3��2CBr-CH2Br��Dת���õ�E��E����������������G��Ӧ��Dˮ������E����EΪ��CH3��2C��OH��-CH2OH��FΪ��CH3��2C��OH��-CHO��F��������G��G��õ�H����HΪ��CH3��2C��OH��-COOH��GΪ��CH3��2C��OH��-COONa��H�����ɻ�������Ӧ����M����MΪ
����CH3��2C=CH2���巢���ӳɷ�Ӧ����DΪ��CH3��2CBr-CH2Br��Dת���õ�E��E����������������G��Ӧ��Dˮ������E����EΪ��CH3��2C��OH��-CH2OH��FΪ��CH3��2C��OH��-CHO��F��������G��G��õ�H����HΪ��CH3��2C��OH��-COOH��GΪ��CH3��2C��OH��-COONa��H�����ɻ�������Ӧ����M����MΪ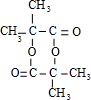 ���ݴ˽��н��
���ݴ˽��н��| 57 |
| 12 |
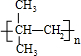 ����CH3��2C=CH2���巢���ӳɷ�Ӧ����DΪ��CH3��2CBr-CH2Br��Dת���õ�E��E����������������G��Ӧ��Dˮ������E����EΪ��CH3��2C��OH��-CH2OH��FΪ��CH3��2C��OH��-CHO��F��������G��G��õ�H����HΪ��CH3��2C��OH��-COOH��GΪ��CH3��2C��OH��-COONa��H�����ɻ�������Ӧ����M����MΪ
����CH3��2C=CH2���巢���ӳɷ�Ӧ����DΪ��CH3��2CBr-CH2Br��Dת���õ�E��E����������������G��Ӧ��Dˮ������E����EΪ��CH3��2C��OH��-CH2OH��FΪ��CH3��2C��OH��-CHO��F��������G��G��õ�H����HΪ��CH3��2C��OH��-COOH��GΪ��CH3��2C��OH��-COONa��H�����ɻ�������Ӧ����M����MΪ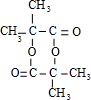 ��
��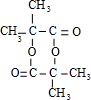 ��
��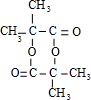 ��
��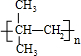 ����Ӧ����ʽΪ��n��CH3��2C=CH2
����Ӧ����ʽΪ��n��CH3��2C=CH2| ���� |
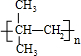 ��
��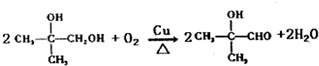 ��
��| ���� |
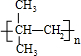 ��
��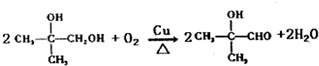 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����������ȼ��ʱ�õ�һ�ָƵ������ᄃ�壬��ṹ��ͼ��ʾ���ɴ˿��жϸøƵ�������Ļ�ѧʽΪ
����������ȼ��ʱ�õ�һ�ָƵ������ᄃ�壬��ṹ��ͼ��ʾ���ɴ˿��жϸøƵ�������Ļ�ѧʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����֬�Ǹ߷��ӻ�̨�ˮ������ɸ��ͺ�֬���� |
| B��������ע��Һ���ܲ��������ЧӦ�������ڽ��� |
| C��ú�к��е�ú���ͣ�����ú������ |
| D������ϩ���ϵ��ϻ�����Ϊ�����˼ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����A��B��C�������ʣ�����ͬһ��Ԫ�أ�һ����������ת����ϵ���£����ֲ�������ȥ����
����A��B��C�������ʣ�����ͬһ��Ԫ�أ�һ����������ת����ϵ���£����ֲ�������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ʵ��������ϩ |
B�� ʵ�����ư� |
C�� ʯ������ |
D�� ʵ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����������A��C��D�dz������壬A�ǵ��¡�����ЧӦ������Ҫ���ʣ�B�dz�����ɫҺ�壬C��ʹʪ��ĺ�ɫʯ����ֽ������F�ǰ�ɫ������G�Ǻ��ɫ������X��������A��B��C�����ʵ���֮��Ϊ1��1��1��
�й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����������A��C��D�dz������壬A�ǵ��¡�����ЧӦ������Ҫ���ʣ�B�dz�����ɫҺ�壬C��ʹʪ��ĺ�ɫʯ����ֽ������F�ǰ�ɫ������G�Ǻ��ɫ������X��������A��B��C�����ʵ���֮��Ϊ1��1��1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com