
ΓΨΧβΡΩΓΩ«κΡψάϊ”ΟΥυ―ßΒΡΜ·―ßΖ¥”Π‘≠άμΫβΨωœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©œ¬Ν–»ή“Κ‘ΎΩ’Τχ÷–Φ”»»’τΗ…Θ§≤Δ«“ΉΤ…’÷°ΚσΘ§ΥυΒΟΙΧΧε»‘ΈΣ‘≠»ή“Κ÷–ΒΡ»ή÷ ΒΡ «__________ΓΘ
AΘ°FeCl3BΘ°NaClOCΘ°Fe2(SO4)3DΘ°K2SO3
Θ®2Θ©±Κ÷ΤΗβΒψΑ―NaHCO3ΉςΈΣΖΔΫΆΖέ Ι”ΟΘ§ΆυΆυΧμΦ”…ΌΝΩΒΡΟςΖ·Θ§ΨΆ «άϊ”ΟΟςΖ·÷–ΒΡAl3+”κHCO3-ΖΔ…ζΥΪΥ°ΫβΘ§–¥≥ωάκΉ”ΖΫ≥Χ Ϋ ____________________________
Θ®3Θ©≥ΘΈ¬œ¬Θ§”–0.1mol/LΒΡ―ΈΥαΘ§”…Υ°Βγάκ≥ωΒΡc(H+)= __________ Θ§ΗΟ―ΈΥα”κ0.04mol/LΒΡBa(OH)2Β»ΧεΜΐΜλΚœΘ§ΜλΚœΚσΒΡpH÷Β________
Θ®4Θ©≥ΘΈ¬œ¬Θ§Έο÷ ΒΡΝΩ≈®Ε»ΨυΈΣ0.1 molΓΛL-1ΒΡœ¬Ν–»ή“ΚΘΚ
ΔΌNa2CO3»ή“Κ ΔΎNaOH»ή“Κ ΔέCH3COONa»ή“Κ ΔήNH4Cl»ή“Κ ΔίNaNO3Θ§»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡ≈≈Ν–Υ≥–ρ «__________________
Θ®5Θ© Β―ιΘΚΔΌ0.1 molΓΛLΘ≠1 AgNO3»ή“ΚΚΆ0.1 molΓΛLΘ≠1NaCl»ή“ΚΒ»ΧεΜΐΜλΚœΒΟΒΫΉ«“ΚaΘ§Ιΐ¬ΥΒΟΒΫ¬Υ“ΚbΚΆΑΉ…Ϊ≥ΝΒμcΘΜΔΎœρ¬Υ“Κb÷–ΒΈΦ”0.1 molΓΛLΘ≠1 KI»ή“ΚΘ§≥ωœ÷ΜκΉ«ΘΜΔέœρ≥ΝΒμc÷–ΒΈΦ”0.1 molΓΛLΘ≠1KI»ή“ΚΘ§≥ΝΒμ±δΈΣΜΤ…ΪΓΘ
A. –¥≥ωAgCl≥ΝΒμ»ήΫβΤΫΚβΖΫ≥Χ Ϋ______________________
B. Δέ÷–―’…Ϊ±δΜ·ΥΒΟςKsp(AgCl) ____ Ksp(AgI)(ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΓΔΓΑ=Γ±)
ΓΨ¥πΑΗΓΩ C Al3++3HCO3-==Al(OH)3Γΐ+3CO2Γϋ 10-13mol/L 2 ΔΎ>ΔΌ>Δέ>Δί>Δή AgCl(s) ![]() Ag+(aq)+Cl-(aq) >
Ag+(aq)+Cl-(aq) >
ΓΨΫβΈωΓΩ ‘ΧβΖ÷ΈωΘΚ±ΨΧβΩΦ≤ι―Έ»ή“ΚΦ”»»’τΗ…≤ΔΉΤ…’Κσ≤ζΈοΒΡ≈–ΕœΘ§ΥΪΥ°ΫβΖ¥”ΠάκΉ”ΖΫ≥Χ ΫΒΡ ι–¥Θ§Υ°ΒγάκcΘ®H+Θ©ΚΆΥαΦνΜλΚœΚσpHΒΡΦΤΥψΘ§Β»Έο÷ ΒΡΝΩ≈®Ε»≤ΜΆ§»ή“ΚpH¥σ–ΓΒΡ≈–ΕœΘ§≥ΝΒμΒΡΉΣΜ·ΓΘ
Θ®1Θ©AœνΘ§FeCl3‘Ύ»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβFeCl3+3H2O![]() FeΘ®OHΘ©3+3HClΘ§Φ”»» ±”…”ΎHClΒΡΜ”ΖΔΘ§Υ°ΫβΤΫΚβ’ΐœρ“ΤΕ·Θ§Ήν÷’FeCl3Άξ»ΪΥ°Ϋβ≥…FeΘ®OHΘ©3Θ§ΉΤ…’ ±FeΘ®OHΘ©3Ζ÷Ϋβ≥…Fe2O3ΚΆH2OΘ§Ήν÷’ΥυΒΟΙΧΧεΈΣFe2O3ΘΜBœνΘ§NaClO‘Ύ»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβNaClO+H2O
FeΘ®OHΘ©3+3HClΘ§Φ”»» ±”…”ΎHClΒΡΜ”ΖΔΘ§Υ°ΫβΤΫΚβ’ΐœρ“ΤΕ·Θ§Ήν÷’FeCl3Άξ»ΪΥ°Ϋβ≥…FeΘ®OHΘ©3Θ§ΉΤ…’ ±FeΘ®OHΘ©3Ζ÷Ϋβ≥…Fe2O3ΚΆH2OΘ§Ήν÷’ΥυΒΟΙΧΧεΈΣFe2O3ΘΜBœνΘ§NaClO‘Ύ»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβNaClO+H2O![]() NaOH+HClOΘ§HClO ή»»Ζ÷ΫβΘΚ2HClO
NaOH+HClOΘ§HClO ή»»Ζ÷ΫβΘΚ2HClO![]() 2HCl+O2ΓϋΘ§Υ°ΫβΤΫΚβ’ΐœρ“ΤΕ·Θ§NaOH+HCl=NaCl+H2OΘ§’τΗ…ΉΤ…’ΥυΒΟΙΧΧεΈΣNaClΘΜCœνΘ§Fe2Θ®SO4Θ©3‘Ύ»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβFe2Θ®SO4Θ©3+6H2O
2HCl+O2ΓϋΘ§Υ°ΫβΤΫΚβ’ΐœρ“ΤΕ·Θ§NaOH+HCl=NaCl+H2OΘ§’τΗ…ΉΤ…’ΥυΒΟΙΧΧεΈΣNaClΘΜCœνΘ§Fe2Θ®SO4Θ©3‘Ύ»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβFe2Θ®SO4Θ©3+6H2O![]() 2FeΘ®OHΘ©3+3H2SO4Θ§ΒΪ”…”ΎH2SO4ΈΣ≤ΜΜ”ΖΔ–‘ΥαΘ§Υυ“‘Φ”»»’τΗ…≤ΔΉΤ…’ΚσΥυΒΟΙΧΧεΈΣFe2Θ®SO4Θ©3ΘΜDœνΘ§K2SO3‘ΎΦ”»»’τΗ…Ιΐ≥Χ÷–±Μ―θΤχ―θΜ·≥…K2SO4Θ®Ζ¥”ΠΈΣ2K2SO3+O2=2K2SO4Θ©Θ§ΉΤ…’ΚσΥυΒΟΙΧΧεΈΣK2SO4ΘΜ‘ΎΩ’Τχ÷–Φ”»»’τΗ…≤ΔΉΤ…’ΚσΥυΒΟΙΧΧε»‘ΈΣ‘≠»ή÷ ΒΡ «Fe2Θ®SO4Θ©3Θ§¥πΑΗ―ΓCΓΘ
2FeΘ®OHΘ©3+3H2SO4Θ§ΒΪ”…”ΎH2SO4ΈΣ≤ΜΜ”ΖΔ–‘ΥαΘ§Υυ“‘Φ”»»’τΗ…≤ΔΉΤ…’ΚσΥυΒΟΙΧΧεΈΣFe2Θ®SO4Θ©3ΘΜDœνΘ§K2SO3‘ΎΦ”»»’τΗ…Ιΐ≥Χ÷–±Μ―θΤχ―θΜ·≥…K2SO4Θ®Ζ¥”ΠΈΣ2K2SO3+O2=2K2SO4Θ©Θ§ΉΤ…’ΚσΥυΒΟΙΧΧεΈΣK2SO4ΘΜ‘ΎΩ’Τχ÷–Φ”»»’τΗ…≤ΔΉΤ…’ΚσΥυΒΟΙΧΧε»‘ΈΣ‘≠»ή÷ ΒΡ «Fe2Θ®SO4Θ©3Θ§¥πΑΗ―ΓCΓΘ
Θ®2Θ©Al3+Υ°ΫβΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl3++3H2O![]() AlΘ®OHΘ©3+3H+Θ§HCO3-Υ°ΫβΒΡάκΉ”ΖΫ≥Χ ΫΈΣHCO3-+H2O
AlΘ®OHΘ©3+3H+Θ§HCO3-Υ°ΫβΒΡάκΉ”ΖΫ≥Χ ΫΈΣHCO3-+H2O![]() H2CO3+OH-Θ§Al3+”κHCO3-ΖΔ…ζΥΪΥ°ΫβΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl3++3HCO3-=AlΘ®OHΘ©3Γΐ+3CO2ΓϋΓΘ
H2CO3+OH-Θ§Al3+”κHCO3-ΖΔ…ζΥΪΥ°ΫβΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣAl3++3HCO3-=AlΘ®OHΘ©3Γΐ+3CO2ΓϋΓΘ
Θ®3Θ©≥ΘΈ¬œ¬0.1mol/L―ΈΥα÷–cΘ®H+Θ©=0.1mol/LΘ§cΘ®OH-Θ©=1![]() 10-13mol/LΘ§―ΈΥα÷–OH-»Ϊ≤Ωά¥Ή‘Υ°ΒΡΒγάκΘ§‘ρΥ°Βγάκ≥ωΒΡcΘ®H+Θ©=Υ°Βγάκ≥ωΒΡcΘ®OH-Θ©=1
10-13mol/LΘ§―ΈΥα÷–OH-»Ϊ≤Ωά¥Ή‘Υ°ΒΡΒγάκΘ§‘ρΥ°Βγάκ≥ωΒΡcΘ®H+Θ©=Υ°Βγάκ≥ωΒΡcΘ®OH-Θ©=1![]() 10-13mol/LΓΘ―ΈΥα»ή“Κ÷–nΘ®H+Θ©=0.1VΘ§BaΘ®OHΘ©2»ή“Κ÷–nΘ®OH-Θ©=0.08VΘ§―ΈΥα”κBaΘ®OHΘ©2»ή“ΚΜλΚœΖΔ…ζΒΡάκΉ”Ζ¥”ΠΈΣH++OH-=H2OΘ§Ω…ΦϊΖ¥”ΠΚσΥαΙΐΝΩΘ§»ή“Κ≥ Υα–‘Θ§cΘ®H+Θ©ΙΐΝΩ=Θ®0.1V-0.08VΘ©
10-13mol/LΓΘ―ΈΥα»ή“Κ÷–nΘ®H+Θ©=0.1VΘ§BaΘ®OHΘ©2»ή“Κ÷–nΘ®OH-Θ©=0.08VΘ§―ΈΥα”κBaΘ®OHΘ©2»ή“ΚΜλΚœΖΔ…ζΒΡάκΉ”Ζ¥”ΠΈΣH++OH-=H2OΘ§Ω…ΦϊΖ¥”ΠΚσΥαΙΐΝΩΘ§»ή“Κ≥ Υα–‘Θ§cΘ®H+Θ©ΙΐΝΩ=Θ®0.1V-0.08VΘ©![]() Θ®V+VΘ©=0.01mol/LΘ§pH=-lg cΘ®H+Θ©ΙΐΝΩ=2ΓΘ
Θ®V+VΘ©=0.01mol/LΘ§pH=-lg cΘ®H+Θ©ΙΐΝΩ=2ΓΘ
Θ®4Θ©Na2CO3ΓΔCH3COONaΕΦ τ”Ύ«ΩΦν»θΥα―ΈΘ§Na2CO3ΓΔCH3COONa»ή“ΚΕΦ≥ Φν–‘Θ§”…”ΎΥα–‘ΘΚCH3COOH![]()
![]() HCO3-Θ§Υυ“‘CO32-ΒΡΥ°ΫβΡήΝΠ¥σ”ΎCH3COO-Θ§‘ρΒ»Έο÷ ΒΡΝΩ≈®Ε»ΒΡNa2CO3»ή“ΚΒΡpH¥σ”ΎCH3COONaΘΜNaOH τ”Ύ«ΩΦν»ή“ΚΘ§Άξ»ΪΒγάκΘ§pHΉν¥σΘΜNH4Cl τ”Ύ«ΩΥα»θΦν―ΈΘ§NH4Cl»ή“Κ≥ Υα–‘Θ§pHΉν–ΓΘΜNaNO3 τ”Ύ«ΩΥα«ΩΦν―ΈΘ§NaNO3»ή“Κ≥ ÷––‘ΘΜ »ή“ΚpH”…¥σΒΫ–ΓΒΡΥ≥–ρ «ΔΎ
HCO3-Θ§Υυ“‘CO32-ΒΡΥ°ΫβΡήΝΠ¥σ”ΎCH3COO-Θ§‘ρΒ»Έο÷ ΒΡΝΩ≈®Ε»ΒΡNa2CO3»ή“ΚΒΡpH¥σ”ΎCH3COONaΘΜNaOH τ”Ύ«ΩΦν»ή“ΚΘ§Άξ»ΪΒγάκΘ§pHΉν¥σΘΜNH4Cl τ”Ύ«ΩΥα»θΦν―ΈΘ§NH4Cl»ή“Κ≥ Υα–‘Θ§pHΉν–ΓΘΜNaNO3 τ”Ύ«ΩΥα«ΩΦν―ΈΘ§NaNO3»ή“Κ≥ ÷––‘ΘΜ »ή“ΚpH”…¥σΒΫ–ΓΒΡΥ≥–ρ «ΔΎ![]() ΔΌ
ΔΌ![]() Δέ
Δέ![]() Δί
Δί![]() ΔήΓΘ
ΔήΓΘ
Θ®5Θ©A.AgCl≥ΝΒμ»ήΫβΤΫΚβΖΫ≥Χ ΫΈΣAgClΘ®sΘ©![]() Ag+Θ®aqΘ©+Cl-Θ®aqΘ©ΓΘ
Ag+Θ®aqΘ©+Cl-Θ®aqΘ©ΓΘ
B.ΔΌ0.1 mol/L AgNO3»ή“ΚΚΆ0.1 mol/L NaCl»ή“ΚΒ»ΧεΜΐΜλΚœ«ΓΚΟΆξ»ΪΖ¥”ΠΒΟΒΫΒΡΑΉ…Ϊ≥ΝΒμcΈΣAgClΘ§œρAgCl÷–Φ”»κKI»ή“ΚΘ§≥ΝΒμ±δΈΣΜΤ…ΪΘ§ΥΒΟςAgClΉΣΜ·ΈΣAgIΘ§ΗυΨί≥ΝΒμΉΣΜ·ΒΡ“ΜΑψ‘≠‘ρΒΟ≥ωKspΘ®AgClΘ©![]() KspΘ®AgIΘ©ΓΘ
KspΘ®AgIΘ©ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ “―÷Σ≥ΘΈ¬œ¬≤ΩΖ÷»θΒγΫβ÷ ΒΡΒγάκΤΫΚβ≥Θ ΐ»γœ¬±μΘΚ
Μ·―ß Ϋ | HF | HClO | H2CO3 | NH3ΓΛH2O |
Βγάκ≥Θ ΐ | 6.8ΓΝ104 | 4.7ΓΝ108 | K1=4.3ΓΝ107 K2=5.6ΓΝ1011 | Kb=1.7ΓΝ105 |
Θ®1Θ©≥ΘΈ¬œ¬Θ§PHœύΆ§ΒΡ»ΐ÷÷»ή“ΚΔΌNaF»ή“Κ ΔΎNaClO»ή“Κ ΔέNa2CO3»ή“ΚΘ§ΤδΈο÷ ΒΡΝΩ»ήΕ»”…¥σΒΫ–ΓΒΡΥ≥–ρ «___________Θ®Χν–ρΚ≈Θ©
Θ®2Θ©25ΓψC ±Θ§PH=4ΒΡNH4Cl»ή“Κ÷–ΗςάκΉ”≈®Ε»ΒΡ¥σ–ΓΙΊœΒΈΣ___________________
Θ®3Θ©0.1 mol/LΒΡNaClO»ή“ΚΚΆ0.1 mol/LΒΡNaHCO3»ή“Κ÷–Θ§c(ClO-)________ c(HCO3-)Θ®ΧνΓΑ>Θ§<Θ§=Γ±Θ©Ω… Ι…œ ωΝΫ÷÷»ή“ΚPHœύΒ»ΒΡΖΫΖ® «___________Θ®Χν¥ζΚ≈Θ©
a.œρNaClO»ή“Κ÷–Φ” ΝΩΒΡΥ° b.œρNaClO»ή“Κ÷–Φ” ΝΩΒΡNaOH
c.œρNaHCO3»ή“Κ÷–Φ” ΝΩΒΡΥ° d. œρNaHCO3»ή“Κ÷–Φ” ΝΩΒΡNaOH
Θ®4Θ©œρNaClO»ή“Κ÷–Ά®»κ…ΌΝΩΒΡCO2Θ§ΥυΖΔ…ζΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_________
Θ®5Θ©≥ΘΈ¬œ¬Θ§0.1mol/LΒΡΑ±Υ°ΚΆ0.1mol/LΒΡNH4Cl»ή“ΚΒ»ΧεΜΐΜλΚœΘ§≈–ΕœΜλΚœ»ή“ΚΒΡΥαΦν–‘____________(ΧνΓΑΥα–‘Γ±ΓΑΦν–‘Γ±ΓΑ÷––‘Γ±)
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–”–ΙΊΈο÷ Ήι≥…ΒΡΥΒΖ®’ΐ»ΖΒΡ «Θ®Θ©
A.Έο÷ Ψυ «”…Ζ÷Ή”ΙΙ≥…Θ§Ζ÷Ή”Ψυ «”…‘≠Ή”ΙΙ≥…
B.÷Μ”…“Μ÷÷‘ΣΥΊΉι≥…ΒΡΈο÷ “ΜΕ® «ΒΞ÷
C.Ϋπ τ―θΜ·ΈοΕΦ «Φν–‘―θΜ·Έο
D.ΝρΥα «¥ΩΨΜΈοΘ§―ΈΥα «ΜλΚœΈο
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘Ύ«ΩΥα–‘»ή“Κ÷–Ρή¥σΝΩΙ≤¥φΒΡΈό…ΪΆΗΟςάκΉ”Ήι «Θ® Θ©
A. KΘΪΓΔNaΘΪΓΔCO32Θ≠ΓΔClΘ≠ B. NH4ΘΪΓΔNa °ΓΔBrΘ≠ΓΔCu2ΘΪ
C. Mg2ΘΪΓΔNaΘΪΓΔNO3Θ≠ΓΔSO42Θ≠ D. Na °ΓΔBa2ΘΪΓΔOHΘ≠ΓΔSO42Θ≠
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ–¬–Άκ¬(N2H4)-Ω’Τχ»ΦΝœΒγ≥Ί «“Μ÷÷ΜΖ±Θ–ΆΦν–‘»ΦΝœΒγ≥ΊΘ§ΤδΙΛΉς‘≠άμ «N2H4+O2=N2+2H2O,Βγ≥ΊΖ≈Βγ ±Θ§ΒγΉ””…bΦΪΨ≠ΆβΒγ¬ΖΝςœρaΦΪΓΘœ¬Ν–”–ΙΊΗΟ»ΦΝœΒγ≥ΊΒΡΥΒΖ®’ΐ»ΖΒΡ «(ΓΓΓΓ)
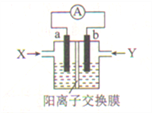
A. ΗΟΉΑ÷Ο÷–Θ§XΈΣN2H4Θ§YΈΣΩ’Τχ
B. ΗΚΦΪΒΡΒγΦΪΖ¥”Π ΫΘΚO2+2H2O+4e-=4OH-
C. Βγ≥ΊΙΛΉς ±Θ§bΦΪΗΫΫϋ»ή“ΚpH…ΐΗΏ
D. »τΒγΫβ÷ »ή“ΚΈΣKOH»ή“ΚΘ§‘ρK+”…”“≥ΊΆ®ΙΐΫΜΜΜΡΛœρΉσ≥Ί«®“Τ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬ΆΦ±μ ΨΒΡΖ¥”ΠΙΊœΒ÷–Θ§≤ΩΖ÷≤ζΈο±Μ¬‘»ΞΓΘ“―÷Σ2 molΑΉ…ΪΙΧΧεΖέΡ©X ή»»Ζ÷ΫβΘ§Μ÷Η¥ΒΫ “Έ¬…ζ≥…ΑΉ…ΪΙΧΧεAΓΔΈό…Ϊ“ΚΧεBΚΆΈό…ΪΤχΧεCΗς1 molΓΘXΓΔEΓΔGΒΡ―φ…ΪΖ¥”ΠΨυΈΣΜΤ…ΪΓΘ

ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©–¥≥ωœ¬Ν–Έο÷ ΒΡΜ·―ß ΫΘΚG_____________ D_____________
Θ®2Θ©–¥≥ωG”κCΖ¥”Π…ζ≥…DΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΘΚ____________________________
Θ®3Θ©–¥≥ωXΘΪEΓζAΒΡάκΉ”ΖΫ≥Χ ΫΘΚ______________________________________
Θ®4Θ©–¥≥ωC”κNa2O2Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_____________________________Θ§»τ”–0.2 mol Na2O2≤ΈΦ”Ζ¥”Π‘ρΉΣ“ΤΒΡΒγΉ” ΐΡΩΈΣ_____________________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΥΒΖ®¥μΈσΒΡ «
AΘ°»»Μ·―ßΖΫ≥Χ ΫΗςΈο÷ «ΑΒΡΜ·―ßΦΤΝΩ ΐ≤Μ±μ ΨΖ÷Ή”Ηω ΐΘ§÷Μ¥ζ±μΈο÷ ΒΡΝΩ
BΘ°»»Μ·―ßΖΫ≥Χ ΫΈ¥ΉΔΟςΈ¬Ε»ΚΆ―Ι«Ω ±Θ§ΠΛH±μ Ψ±ξΉΦΉ¥Ωωœ¬ΒΡ ΐΨί
CΘ°Ά§“ΜΜ·―ßΖ¥”ΠΘ§Μ·―ßΦΤΝΩ ΐ≤ΜΆ§Θ§ΠΛH≤ΜΆ§Θ§Μ·―ßΦΤΝΩ ΐœύΆ§ΕχΉ¥Χ§≤ΜΆ§Θ§ΠΛH“≤≤ΜœύΆ§
DΘ°Μ·―ßΖ¥”ΠΙΐ≥ΧΥυΈϋ ’ΜρΖ≈≥ωΒΡ»»ΝΩ”κ≤ΈΦ”Ζ¥”ΠΒΡΈο÷ ΒΡΈο÷ ΒΡΝΩ≥…’ΐ±»
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥Ά§―ß‘Ύ―ßœΑ‘ΣΥΊΜ·ΚœΈο÷Σ ΕΒΡΙΐ≥Χ÷–Θ§ΖΔœ÷Κ§”–œύΆ§‘ΣΥΊΒΡΈο÷ Φδ‘Ύ“ΜΕ®ΧθΦΰœ¬¥φ‘ΎΉΣΜ·Ιφ¬…Θ§Μφ÷Τ≥ω»γœ¬ΉΣΜ·ΙΊœΒΆΦΓΘ
![]()
«κΜΊ¥πΘΚ
Θ®1Θ©»τAΈΣΡή Ι Σ»σΒΡΚλ…Ϊ ·»ο ‘÷Ϋ±δάΕΒΡΤχΧεΘ§CΈΣΚλΉΊ…ΪΤχΧεΓΘ
ΔΌAΓΔBΓΔCΓΔD÷–ΨυΚ§”–ΒΡ‘ΣΥΊ «_________________(Χν‘ΣΥΊΖϊΚ≈)
ΔΎAΓζBΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «________________________ΓΘ
Θ®2Θ©»τAΈΣΫπ τΒΞ÷ Θ§CΈΣΒ≠ΜΤ…ΪΙΧΧεΓΘ
ΔΌAΓΔBΓΔCΓΔD÷–ΨυΚ§”–ΒΡ‘ΣΥΊ «_________________(Χν‘ΣΥΊΖϊΚ≈)
ΔΎCΓζDΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «________________________ΓΘΗΟΖ¥”Π÷–Θ§ΖΔ…ζ―θΜ·Ζ¥”ΠΚΆΜΙ‘≠Ζ¥”ΠΒΡΈο÷ ΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ_________________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥ΩΤ―––ΓΉι≤…”Ο»γœ¬ΖΫΑΗΜΊ ’“Μ÷÷Ιβ≈ΧΫπ τ≤ψ÷–ΒΡ…ΌΝΩAg(Ϋπ τ≤ψ÷–ΤδΥϊΫπ τΚ§ ΝΩΙΐΒΆ,Ε‘ Β―ιΒΡ”ΑœλΩ…Κω¬‘)ΓΘ

“―÷Σ:ΔΌNaClO»ή“Κ‘Ύ ή»»ΜρΥα–‘ΧθΦΰœ¬“ΉΖ÷Ϋβ,»γ: 3NaClO![]() 2NaCl+NaClO3
2NaCl+NaClO3
ΔΎAgClΩ…»ή”ΎΑ±Υ°:AgCl+2NH3ΓΛH2O![]() Ag(NH3) 2++ Cl- +2H2O
Ag(NH3) 2++ Cl- +2H2O
Δέ≥ΘΈ¬ ± N2H4ΓΛH2O(Υ°Κœκ¬)‘ΎΦν–‘ΧθΦΰœ¬ΡήΜΙ‘≠ Ag(NH3) 2+ :
4 Ag(NH3) 2++N2H4ΓΛH2O![]() 4AgΓΐ+ N2Γϋ+ 4
4AgΓΐ+ N2Γϋ+ 4![]() + 4NH3Γϋ+H2O
+ 4NH3Γϋ+H2O
Θ®1Θ©ΓΑ―θΜ·Γ±ΫΉΕΈ–η‘Ύ 80ΓφΧθΦΰœ¬Ϋχ––, “ΥΒΡΦ”»»ΖΫ ΫΈΣ__________________ΓΘ
Θ®2Θ©NaClO »ή“Κ”κ Ag Ζ¥”ΠΒΡ≤ζΈοΈΣ AgClΓΔNaOH ΚΆ O2 ,ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_____________ΓΘ HNO3“≤Ρή―θΜ·Ag,¥”Ζ¥”Π≤ζΈοΒΡΫ«Ε»Ζ÷Έω,“‘HNO3¥ζΧφNaClOΒΡ»±Βψ «___________________ΓΘ
Θ®3Θ©ΈΣΧαΗΏAgΒΡΜΊ ’¬ ,–ηΕ‘ΓΑΙΐ¬ΥΔρΓ±ΒΡ¬Υ‘ϋΫχ––œ¥Β”,≤Δ_______________________ΓΘ
Θ®4Θ©»τ Γ¬‘ΓΑΙΐ¬ΥΔώΓ±,÷±Ϋ”œρά以ΚσΒΡΖ¥”Π»ίΤς÷–ΒΈΦ”10%Α±Υ°,‘ρ–η“Σ‘ωΦ”Α±Υ°ΒΡ”ΟΝΩ,≥ΐ“ρΙΐΝΩNaClO”κNH3ΓΛH2OΖ¥”ΠΆβ(ΗΟΧθΦΰœ¬NaClO3”κNH3ΓΛH2O≤ΜΖ¥”Π),ΜΙ“ρΈΣ___________________________ΓΘ
Θ®5Θ©«κ…ηΦΤ¥”ΓΑΙΐ¬ΥΔρΓ±ΚσΒΡ¬Υ“Κ÷–Μώ»ΓΒΞ÷ AgΒΡ Β―ιΖΫΑΗ:________________________( Β―ι÷––κ Ι”ΟΒΡ ‘ΦΝ”–: 2 molΓΛL-1Υ°Κœκ¬»ή“Κ,1 molΓΛL-1H2SO4 )ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com