
����Ŀ�� ��֪�����²���������ʵĵ���ƽ�ⳣ�����±���
��ѧʽ | HF | HClO | H2CO3 | NH3��H2O |
���볣�� | 6.8��104 | 4.7��108 | K1=4.3��107 K2=5.6��1011 | Kb=1.7��105 |
��1�������£�PH��ͬ��������Һ��NaF��Һ ��NaClO��Һ ��Na2CO3��Һ�������ʵ����ܶ��ɴ�С��˳����___________������ţ�
��2��25��Cʱ��PH=4��NH4Cl��Һ�и�����Ũ�ȵĴ�С��ϵΪ___________________
��3��0.1 mol/L��NaClO��Һ��0.1 mol/L��NaHCO3��Һ�У�c(ClO-)________ c(HCO3-)������>��<��=������ʹ����������ҺPH��ȵķ�����___________������ţ�
a.��NaClO��Һ�м�������ˮ b.��NaClO��Һ�м�������NaOH
c.��NaHCO3��Һ�м�������ˮ d. ��NaHCO3��Һ�м�������NaOH
��4����NaClO��Һ��ͨ��������CO2�������������ӷ���ʽΪ_________
��5�������£�0.1mol/L�İ�ˮ��0.1mol/L��NH4Cl��Һ�������ϣ��жϻ����Һ�������____________(����ԡ������ԡ������ԡ�)
���𰸡� ��>��>�� c(Cl-)> c(NH4+) > c(H+)> c(OH-) < a��d ClO-+CO2+H2O=HClO+HCO3- ����
����������1�������ʵ���Ũ�ȵ�������Һ���������ˮ��̶�Խ������Һ��pHԽ����ĵ��볣��ԽС���������ˮ��̶�Խ�����Գ����£�pH��ͬ��������Һ��NaF��Һ ��NaClO��Һ ��Na2CO3��Һ�������ʵ����ܶ��ɴ�С��˳������>��>������2��25��Cʱ��pH=4��NH4Cl��Һ�У�笠�����ˮ�⣬Ũ��С�������ӣ���Һ�����ԣ�������Ũ�ȴ�������������Ũ�ȣ��ʸ�����Ũ�ȵĴ�С��ϵΪc(Cl-)> c(NH4+) > c(H+)> c(OH-)����3�����ݵ��볣����֪��HClO ���볣������H2CO3�ĵ��볣�������Ũ�ȵ�NaClO��Һˮ��̶ȴ���NaHCO3��Һ��ˮ��̶ȣ���0.1 mol/L��NaClO��Һ��0.1 mol/L��NaHCO3��Һ�У�c(ClO-)<c(HCO3-)��ʹ����������ҺpH��ȣ�������ǰ������������Ũ�ȼ�С��a.��NaClO��Һ�м�������ˮ��Ũ�ȼ�С������������Ũ�ȼ�С�����У�b.��NaClO��Һ�м�������NaOH����Һ������������Ũ���������У�c.��NaHCO3��Һ�м�������ˮ������������Ũ�ȼ�С�������У�d. ��NaHCO3��Һ�м�������NaOH����Һ������������Ũ�������У���ѡad����4��������ĵ��볣����֪�����߷�Ӧ����̼�����ƺʹ����ᣬ���ӷ���ʽΪClO-+CO2+H2O=HClO+HCO3-����5�������£�0.1mol/L�İ�ˮ��0.1mol/L��NH4Cl��Һ�������ϣ�����c(NH4+)=c(NH3��H2O)����Kb= =
= ![]() =1.7��105>107����Һ�ʼ��ԡ�
=1.7��105>107����Һ�ʼ��ԡ�


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ��������������Ȼ�������٣�ȴ��ά���������������ȱ�ٵġ��ù۵����ͨ��������һʵ���õ�֤������ ��
A. ȱMgʱҶƬ��� B. �Ͳ�ȱ��Bʱֻ���������
C. ����ѪҺ���κ���̫�ͻ�鴤 D. ȱP��Ӱ��ATP������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�ס�������װ�ã���ʢ��Һ�����Ũ�Ⱦ���ͬ������������װ�õ�·��ͨ���ĵ��Ӷ���1 molʱ������˵������ȷ����
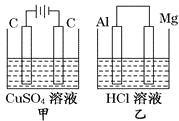
A. ��Һ�������仯����С��������
B. ��ҺpH�仯����С��������
C. ��ͬ�����²�������������V����V��
D. �缫��Ӧʽ������������Cu2+��2e��==Cu�����и�����Mg��2e��==Mg2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ�������������ͼ��ʾ����ش��������⣺
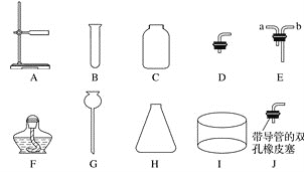
(1)�����������Ϊ__________������G������Ϊ__________��
(2)����B��D��F��ϳ����巢��װ�ã��������ԭ���ķ�Ӧ����һ����__________________��
(3)����C��G��H��I��J(������)��ϳ�һ����ȡ���ռ������װ�ã����װ���ռ��õ��ij������������____________��
(4)����C��Eװ��������������ſ������ռ����壬�������________(����a������b��)���ܿڽ��롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶���HF�ĵ��볣��Ka=4��10-4 ��CaF2���ܶȻ�����Ksp=1.46��10-10���ڸ��¶���ȡŨ��Ϊ0.25 mol��L-1��HF��Ũ��Ϊ0.002 mol��L-1��CaCl2��Һ�������ϡ�����˵����ȷ����
A. ���¶��£�0.25 mol��L-1��HF��Һ��pH=2
B. �����¶Ȼ�����Ũ�ȣ�HF�ĵ���ƽ�ⳣ����������
C. ����Һ��ϲ����������
D. �͵�CaF2��Һ�м�������CaCl2������ܶȻ�����Kspһ����֮ǰ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺

��ѡ�Լ���Na2SO4��Һ��K2CO3��Һ��K2SO4��Һ������
(1)�����ٵ�������________�������ڵ�������____________��
(2)�Լ�a��____________(�ѧʽ����ͬ)���Լ�b��__________,����B��____________��
(3)�����Լ�a��������Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�����Լ�b��������Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(4)�÷����ܷ�ﵽʵ��Ŀ�ģ�__________________________(��ܡ����ܡ�)�������ܣ�Ӧ��θĽ���(���ܣ����ʲ��ûش�)__________��
(5)��Ҫ�ⶨԭ�������BaCl2����������������Ҫȷ���������������⣬���ٻ�Ҫ��õ�������____��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K3[Fe(C2O4)3]��3H2O�������������أ�Ϊ����ɫ���壬������ɹ����ͼ���ش��������⣺
��1��ɹ����ͼʱ����K3[Fe(C2O4)3]��3H2O���й������K3[Fe(CN)6]��ҺΪ��ɫ�������ⷴӦ�Ļ�ѧ����ʽΪ��2K3[Fe(C2O4)3]![]() 2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ijС��Ϊ̽�������������ص��ȷֽ�������ͼ��ʾװ�ý���ʵ�顣

��ͨ�뵪����Ŀ����________________________________________��
��ʵ���й۲쵽װ��B��F�г���ʯ��ˮ������ǣ�װ��E�й����Ϊ��ɫ���ɴ��ж��ȷֽ������һ������___________��___________��
��Ϊ��ֹ������ֹͣʵ��ʱӦ���еIJ�����_____________________________��
����Ʒ��ȫ�ֽ��װ��A�еIJ����ﺬ��FeO��Fe2O3������Fe2O3���ڵķ����ǣ�________________��
��3���ⶨ�����������������ĺ�����
�ٳ���m g��Ʒ����ƿ�У��ܽ���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㡣�ζ��յ��������___________________________��
����������Һ�м������п������Ӧ��ȫ���ˡ�ϴ�ӣ�����Һ��ϴ��Һȫ���ռ�����ƿ�С���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㣬����KMnO4��ҺV mL���þ������������������ı���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��д�����л�ѧ���
��1��H2S����Һ�еĵ��뷽��ʽ��____________________________________________________��
��2�������ӷ���ʽ����NaClO��Һ�Լ��Ե�ԭ��_______________________________________��
��3����ԭ�ӵ���Χ�����Ų�ʽ��________________________��
��4������HF���Ӽ�������________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ��ʯ����ȡ���͵�װ��ʾ��ͼ������ͼʾ�ش��������⡣

(1)ͼ�е��������ԵĴ�����________________��__________________
(2)A������������________��B������������________��
(3)ʵ��ʱ A �г�����ʯ���⣬�����������__________����������__________________��
(4)�ռ������ͺ����ȳ��ƾ��ƻ�����ͣ����ˮ��
______________________________________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com