
����Ŀ���������������ʵ���Һ����NaCl ��NH4Cl ��Na2CO3 ��Al2(SO4)3 ��CH3COOH ��NaHCO3
��1��25��ʱ��0.1mol��L-1����Һ��______�ԣ�0.1mol��L-1����Һ��pH________7���>������������<��������ԭ����______________________________________�������ӷ���ʽ��ʾ����
��2�����£��>������������<�����£�Ũ�Ⱦ�Ϊ0.1mol/L�Ģۺ͢���Һ����������________�����ͬ�����ǡ�����ͬ��������Һ��pH����_________�ޣ��>������������<������
��3��������Һ�������ɲ��������յõ���������____________���ѧʽ����
��4��������0.1 mol/L�Ģ���Һ��ˮϡ�����У����б���ʽ������һ��������_________��
A��c(H��) B��  C��c(H��)��c(OH��)
C��c(H��)��c(OH��)
���𰸡� �� < NH4++H2O![]() NH3��H2O+H+ ��ͬ > Al2(SO4)3 B
NH3��H2O+H+ ��ͬ > Al2(SO4)3 B
����������1���Ȼ��Ʋ�ˮ�⣬25��ʱ��0.1mol��L-1�Ȼ�����Һ�����ԣ�笠�ˮ�⣬��0.1mol��L-1�Ȼ����Һ��pH��7��笠�ˮ������ӷ���ʽΪNH4++H2O![]() NH3��H2O+H+����2��̼������̼���ˮ�⣬��ˮ��ֲ����У�̼�������е�̼��������Ӵ���ˮ��ƽ��͵���ƽ�⣬��Ũ�Ⱦ�Ϊ0.1mol/L������Һ������������ͬ������̼�����ˮ��̶ȴ���̼��������ӣ�����ҺŨ�����ʱ̼������Һ�ļ���ǿ��̼�����ƣ�pH���ۣ��ޣ���3��������ˮ�⣬ˮ�����ȣ����������ɵ��������ѻӷ����ᣬ����������Һ�������ɲ��������յõ���������Ȼ��Al2(SO4)3����4�����������ᣬ���ڵ���ƽ�⣺CH3COOH
NH3��H2O+H+����2��̼������̼���ˮ�⣬��ˮ��ֲ����У�̼�������е�̼��������Ӵ���ˮ��ƽ��͵���ƽ�⣬��Ũ�Ⱦ�Ϊ0.1mol/L������Һ������������ͬ������̼�����ˮ��̶ȴ���̼��������ӣ�����ҺŨ�����ʱ̼������Һ�ļ���ǿ��̼�����ƣ�pH���ۣ��ޣ���3��������ˮ�⣬ˮ�����ȣ����������ɵ��������ѻӷ����ᣬ����������Һ�������ɲ��������յõ���������Ȼ��Al2(SO4)3����4�����������ᣬ���ڵ���ƽ�⣺CH3COOH![]() CH3COO����H����ϡ�ʹٽ����룬��A�������ӵ����ʵ������ӣ���c(H��)���ͣ�A����B��ϡ�����������ӵ����ʵ������ӣ���������ʵ������٣����ֵ
CH3COO����H����ϡ�ʹٽ����룬��A�������ӵ����ʵ������ӣ���c(H��)���ͣ�A����B��ϡ�����������ӵ����ʵ������ӣ���������ʵ������٣����ֵ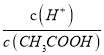 ����B��ȷ��C���¶Ȳ��䣬ˮ�����ӻ��������䣬��c(H��)��c(OH��)���䣬C����ѡB��
����B��ȷ��C���¶Ȳ��䣬ˮ�����ӻ��������䣬��c(H��)��c(OH��)���䣬C����ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽����������X(��������Ԫ��)����ɺ����ʣ���Ʋ��������ʵ�飺

��ش�
(1)X�����Ԫ��ΪH��O��______(��Ԫ�ط��ű�ʾ)����ѧʽΪ________________��
(2)д����һ����Ӧ�ܵõ�X�Ļ�ѧ����ʽ(Ҫ���������ԭ��Ӧ)
_______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ������װ��ͼ�����а�ĤΪ����Ĥ���ס���װ����A��B��a��b��ҺŨ�ȷֱ���MA��MB��Ma��Mb��ʾ���ҡ���װ�÷ֱ��ʾһ��ʱ���ס���װ�õ�״̬��Һ�������ĸ߶ȷֱ�Ϊh1��h2�����A��B��a��b��Ϊ������Һ����MA>MB��Ma��Mb>MA����ﵽƽ���(����)


A. h1>h2��Ma>MbB. h1>h2��Ma<Mb
C. h1<h2��Ma<MbD. h1<h2��Ma>Mb
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽����������̼�Ƿ���ˮ����ʱ���ܺ������Ʒ�Ӧ����ij�����о�С���ͬѧ���������ͼ��ʵ��װ�ã��ֱ�����˼ס�������ʵ�飺

ʵ��ף�����Ķ�����̼�������Ƶķ�Ӧ���ڸ�����Թ�����װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2�����Թ�����װ���Լ�X��K1��K2��ͨ��CO2�������Ӻ������ǵ�ľ�������Թ�����Һ���ϣ��۲쵽ľ������ȼ�������еĵ���ɫû�б仯��
ʵ���ң���ʪ�Ķ�����̼�������Ƶķ�Ӧ�����Թ�����װ���Լ�Y����������ͬʵ��ס��۲쵽ľ����ȼ�������еĵ���ɫ��Ϊ��ɫ��
�Իش��������⣺
(1)Na2O2��ˮ��Ӧ�����ӷ���ʽ____________________________________��
(2)��װ��Na2O2��ͨ��CO2ǰ���ر�K1��K2��Ŀ����____________________��
(3)��ʵ����У��Լ�X��____________����ʵ�����У��Լ�Y��____________���ѧʽ����
(4)������������ʵ�����õ��Ľ�����____________________________________��
(5)�Թ����е�NaOH��Һ��������___________________________________��
(6)Ϊ��ȷ��ʵ���ȷ�ԣ��Ʊ�CO2���õķ�Ӧ�����ѡ��_______(����)��
A������ʯ�� B��С�մ� ��C�����ᡡ D��ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ȡ���Dzⶨ��ֲ����Ʒ�д�֬�������ı���������ԭ����������ͼװ��,����ˮ���ѵ��л��ܼ������������������ȡ��ֲ����Ʒ�еĴ�֬��������i�E����:
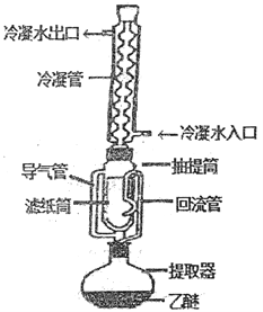
�ٰ�װ:ȡ��ֽ�Ƴ���ֽ��,��������и������������X����ȴ�����¡�Ȼ��������ƿ�г�����ؠ������a������ֽͲ�а���һ��������ϸ����Ʒ����������и������������X����ȴ������,Ȼ��������ƿ�г�����ؠ������b.
����ȡ:��װ����Ʒ����ֽͲ�ó����ӷ������Ͳ��,ע��һ��������ˮ���ѣ�ʹ��ֽͲ��ȫ��û��������,��ͨ����ˮ.���Ȳ������¶ȣ�ʹ�����µε���ˮ���ѳ�����״��������Ͳ�е���ˮ��������ֽ��μ�����ͼ�Ϊֹ(��Լ6h��12h).
�۳���.��ȡ��Ϻ�,�ó�����ȡ����ֽͲ,��ͨ�紦ʹ��ˮ���ѻӷ�����ֽͲ��������и����,��������X����ȴ������.Ȼ��������ƿ�г�����ؠ������c��
�ش���������:
(1)ʵ����ʹ�������ε�����X������________��Ϊ�����������������Ч��,������ȡ���е������ܿ�ѡ��������______��
A.����������![]()
B.ֱ��������![]()
C.����������![]()
(2)��ʵ���б���ʮ��ע�����ѵİ�ȫʹ�ã��粻����������ȡ����ڱ���ͨ��ȡ�Ϊ��ֹ���ѻӷ����������γ�ȼ���������������Ͽ�����һ�����θ���ܣ�����װ���ҩƷΪ____(����ĸ)��
A.����̿ B.��ʯ�� C.P2O5 D.Ũ����
����ˮ�����ڿ����п��������������������������ʱ������ը��������ˮ�������Ƿ��й�������ķ�����__________��
(3)��ʵ����������¶���70�桫80��֮�䣬���ǵ���ȫ�����أ�Ӧ��ȡ�ļ��ȷ�ʽ��____��
�ڵ���ˮ���Ѽ��ȷ��ں�����ͨ��������������������ΪҺ����������У���Һ�泬����������ߴ�ʱ����ȡҺ����������ȡ��(��ƿ)�С����ù�����������������ν��У�����ȡҺ��������ȡ��(��ƿ)����������Ϊ_______��
A.�������� B.���� C.���� D.��Һ
��������ȡ����һ����ȡ����Ƚϣ����ŵ�Ϊ______��
(4)���ݴ���:��Ʒ�д�֬���ٷֺ���____(�>������<����=��)![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ֻ�����������N2��HCl��CO���ѻ����������ͨ��������NaHCO3��Һ�����ȵ�����ͭ��ĩ�����������û�б仯����ͨ�������Ĺ������ƹ��壬���������С�����ͭ��������ַ�Ӧ����������ּ��٣�����������ʣ�ࡣ���¶Ի��������ɵ��жϣ���ȷ����(����)
A. һ��û��N2��HCl��CO��������һ��
B. һ����N2��HCl��CO
C. һ����N2��HCl��CO��������һ��
D. һ����N2��HCl��û��CO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������Ƶ��������ڳ���CO2���壬Ȼ��������ע��������NaOH��Һ�������ý����ܷ�ڡ�����һ��ʱ��ޱ��ڰ������ٹ�һ��ʱ����˵Ĺޱ����¹�������
(1)�ޱ��ڰ������ԭ����_________________________________________��
���ӷ���ʽ����Ϊ_______________________________________________��
(2)���ٹ����ԭ����________________________________________��
���ӷ���ʽ����Ϊ_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش��������⣺
(1)ƫ��������Һ����μ����������������ɹ۲쵽��������____________________����Ӧ�����ӷ���ʽ��____________________________��____________________________��
(2)ƫ��������Һ�г������ϵ�ͨ�������̼���ɹ۲쵽��������________________����Ӧ�����ӷ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��ͨ����������(��Ҫ�ɷ�ΪAl2O3����������Fe2O3��SiO2������)Ϊԭ���Ʊ���ˮ�Ȼ�����2Al2O3��6Cl2===4AlCl3��3O2��
�ش��������⣺
(1)Ϊ�ٽ���Ӧ�Ľ��У�ʵ������������뽹̿����ԭ����____________________��
(2)���뽹̿��Ļ�ѧ��Ӧ�ɱ�ʾΪAl2O3��C��Cl2��,AlCl3��X����������X������
___________________________________________________________��
(3)���ᴿAlCl3�ֲ�Ʒʱ��������������ۣ���ʹ�۵�ϵ͵�FeCl3ת��Ϊ�۵�ϸߵ�FeCl2���Ӷ�������AlCl3�л��������Ȼ���÷�Ӧ�Ļ�ѧ����ʽΪ________________��
(4)��������Ϊԭ�Ͽ���ͨ������;���ᴿ��������

��д����Һ�������ʵĻ�ѧʽ��______________________________��
��д����Һ���м��������ˮ�õ�Al(OH)3�����ӷ���ʽ��_________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com