
����Ŀ����������(��A��)Ԫ�ص��ʼ�����ػ���������ʡ��ϳ�һֱ�����ǻ�ѧ���о����ص㡣�ش��������⣺
��1�����ڼ�������Ԫ��Be��Mg��Ca��Sr��Ba������ԭ�����������ӣ��������ʳʵ����ݼ��仯����___��
A.ԭ�Ӱ뾶 B.���ʵ�Ӳ�� C.��һ������
��2�����������������___ Ԫ���������ơ������й����Ԫ�ص�������ȷ����___���š�
A.������p������Ԫ��
B.�縺�Զ���þ��
C.��һ�����ܶ���þ��
D.�Ȼ����ˮ��ҺpH��С��7
��(NH4)2BeF4�ǹ�ҵ�Ʊ�����������е���Ҫ�м����������Ӻ��еĻ�ѧ������Ϊ__������������ԭ���ӻ���ʽΪ___��
��3��Sr������������Ԫ�أ�SrCO3������Ҫ�Ļ�����֮һ��Sr2+�ĵ����Ų�ʽΪ___�ж�SrCO3���ȷֽ��¶�___������������/��С������CaCO3���ȷֽ��¶ȣ�������____��
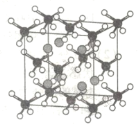
��4��MgH2�ͽ���Ni��һ������������ĥ����ĥ�����Ƶû�ѧʽΪMg2NiH4�Ĵ��⻯��������������ṹ��ͼ��ʾ��
��Mgԭ����Χ�����������ȵ�Niԭ����___�����������߳�Ϊ646pm����Mg��Ni�˼��Ϊ___pm���������С�������λ��![]() ȡ1.73����
ȡ1.73����
�����Ծ���������ܶ���Һ̬���ܶ�֮�ȶ��崢����ϵĴ�����������û�����Ĵ�������Ϊ___���г�����ʽ���ɡ��ٶ��û����������е�H����ȫ���ų���Һ���ܶ�Ϊdg/cm3����NA��������٤��������ֵ����
���𰸡�BC �� BD ���ۼ�����λ�� sp3 [Kr]��[Ar]3d104s24p6 ���� Sr2+�İ뾶����Ca2+����˶�ȡCO![]() �е�O�γ��������������������Ҫ���ߵ��¶� 4 279.40
�е�O�γ��������������������Ҫ���ߵ��¶� 4 279.40 ![]()
��������
��1�����ڼ�������Ԫ��Be��Mg��Ca��Sr��Ba��ͬ����Ԫ�أ�����ԭ�����������ӣ�A��ԭ�Ӱ뾶���� B�����������ܼ�С�����ʵ�Ӳ�ȼ�С��C��ԭ�Ӱ뾶���˶Ե��ӵ�������С����һ�����ܼ�С����ѡBC��
�ʴ�Ϊ��BC��
��2����A��������s����������p������Ԫ�أ���A������
B����λ��þ����һ���ڣ���λ��þͬһ���ڵ��Ҳ࣬�縺�Զ���þ��B���ϣ�
C����λ��þ����һ���ڣ�ԭ�Ӱ뾶С����һ�����ܴ���λ��þͬһ���ڵ��Ҳ࣬þ��3s����ȫ��״̬�����ĵ�һ�����ܱ�þС����C������
D��������ˮ�⣬��Һ�����ԣ�ˮ��ҺpH��С��7����D���ϣ�
�ʴ�Ϊ������BD��
��(NH4)2BeF4�ǹ�ҵ�Ʊ�����������е���Ҫ�м�����������NH4����笠���4������ۼ�,����һ������λ��,���ڹ��ۼ���NH4�����еĻ�ѧ������Ϊ���ۼ�����λ����������BeF![]() ����ԭ����Be,��۲���Ӷ�=4+
����ԭ����Be,��۲���Ӷ�=4+![]() =4���ӻ���ʽΪsp3��
=4���ӻ���ʽΪsp3��
�ʴ�Ϊ�����ۼ�����λ����sp3��
��3�����ǵ������ڵڢ�A��Ԫ�أ�Sr2+�ĵ����Ų�ʽΪ[Kr]��[Ar]3d104s24p6��SrCO3���ȷֽ��¶ȴ��ڣ�����������/��С������CaCO3���ȷֽ��¶ȣ�������Sr2+�İ뾶����Ca2+����˶�ȡCO![]() �е�O�γ��������������������Ҫ���ߵ��¶ȡ�
�е�O�γ��������������������Ҫ���ߵ��¶ȡ�
�ʴ�Ϊ��[Kr]��[Ar]3d104s24p6�����ڣ�Sr2+�İ뾶����Ca2+����˶�ȡCO![]() �е�O�γ��������������������Ҫ���ߵ��¶ȣ�
�е�O�γ��������������������Ҫ���ߵ��¶ȣ�
��4����ͼ��Mgλ��4��NiΧ�ɵ�������������ģ�Mgԭ����Χ�����������ȵ�Niԭ����4�����������߳�Ϊ646pm����Խ���Ϊ646pm��![]() ����Խ���ΪMg��Ni�˼���4������Mg��Ni�˼��Ϊ
����Խ���ΪMg��Ni�˼���4������Mg��Ni�˼��Ϊ![]() =279.40pm��
=279.40pm��
�ʴ�Ϊ��4��279.40��
�ھ�����Mg��8����NiΪ8��![]() +6��
+6��![]() =4��Hԭ��Ϊ4��4=16������������ܶ�Ϊ��
=4��Hԭ��Ϊ4��4=16������������ܶ�Ϊ��![]() ��Һ���ܶ�Ϊdg/cm3�����Ծ���������ܶ���Һ̬���ܶ�֮�ȶ��崢����ϵĴ�����������û�����Ĵ�������Ϊ
��Һ���ܶ�Ϊdg/cm3�����Ծ���������ܶ���Һ̬���ܶ�֮�ȶ��崢����ϵĴ�����������û�����Ĵ�������Ϊ![]() ��
��
�ʴ�Ϊ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʽ��������������Na2SO4��ˮ��ԭ����ͼ��ʾ�����ö��Ե缫��ab��cd��Ϊ���ӽ���Ĥ����ֱ���糡�������£���Ĥ�м��Na+��SO42-��ͨ�����ӽ���Ĥ�������˸��������ӱ��赲���ܽ����м���ҡ�����������ȷ����

A. ͨ����м���ҵ�SO42-����������Ǩ�ƣ���������ҺpH����
B. �÷��ڴ�����Na2SO4��ˮʱ���Եõ�NaOH��H2SO4��Ʒ
C. ������ӦΪ2H2O�C4e�C=O2+4H+����������ҺpH����
D. ����·��ͨ��1mol���ӵĵ���ʱ������0.5mol��O2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ����2L�������������ʵ����ʵ�����ʱ��ı仯��������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽ�ͷ�Ӧ��ʼ��2minĩZ��ƽ����Ӧ����Ϊ(����)
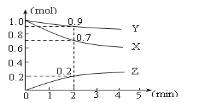
A.3X + Y ![]() 2Z��0.05mol��L-1��min-1B.2X + Y
2Z��0.05mol��L-1��min-1B.2X + Y![]() 2Z��0.1mol��L-1��min-1
2Z��0.1mol��L-1��min-1
C.X + 2Y =Z��0.1mol��L-1��min-1D.X + 3Y =2Z��0.05mol��L-1��min-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�һ�����ܱ������У�����һ������NO(g)������C(s)��������ӦC(s)��2NO(g) ![]() CO2(g)��N2(g)��ƽ��״̬ʱNO(g)�����ʵ���Ũ��[NO]���¶�T�Ĺ�ϵ��ͼ��ʾ��������˵������ȷ����(����)
CO2(g)��N2(g)��ƽ��״̬ʱNO(g)�����ʵ���Ũ��[NO]���¶�T�Ĺ�ϵ��ͼ��ʾ��������˵������ȷ����(����)

A. �÷�Ӧ����H>0
B. ���÷�Ӧ��T1��T2ʱ��ƽ�ⳣ���ֱ�ΪK1��K2����K1<K2
C. ��T2ʱ������Ӧ��ϵ����״̬D�����ʱһ����v��<v��
D. ��T3ʱ�������������ܶȲ��ٱ仯��������жϷ�Ӧ�ﵽƽ��״̬C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ����101 k Paʱ��ǿ����ǿ���ϡ��Һ�����кͷ�Ӧ���к���Ϊ57.3 kJ/mol�������ȼ����Ϊ5518 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ���ǣ�������
A. 2H+(aq) +![]() (aq)+
(aq)+![]() (aq)+2OH
(aq)+2OH![]() (aq)=BaSO4(s)+2H
(aq)=BaSO4(s)+2H![]() O(l);
O(l);![]() H=
H=![]() 57.3 kJ/mol
57.3 kJ/mol
B. KOH(aq)+![]() H
H![]() SO4(aq)=
SO4(aq)=![]() K
K![]() SO4(aq)+H
SO4(aq)+H![]() O(l);
O(l);![]() H=
H=![]() 57.3kJ/mol
57.3kJ/mol
C. C8H18(l)+![]() O
O![]() (g)=8CO
(g)=8CO![]() (g)+ 9H
(g)+ 9H![]() O;
O;![]() H=
H=![]() 5518 kJ/mol
5518 kJ/mol
D. 2C8H18(g)+25O![]() (g)=16CO
(g)=16CO![]() (g)+18H
(g)+18H![]() O(l);
O(l);![]() H=
H=![]() 5518 kJ/mol
5518 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ��2NO2![]() 2NO+O2���ܱ�������Ӧ���ﵽƽ��״̬�ı�־�ǣ���
2NO+O2���ܱ�������Ӧ���ﵽƽ��״̬�ı�־�ǣ���
��1����λʱ��������n mol O2��ͬʱ����2n mol NO2
��2����λʱ��������n mol O2��ͬʱ����2n mol NO
��3����NO2��NO��O2�����ʵ���Ũ�ȱ仯��ʾ��Ӧ���ʵı�Ϊ2:2:1��״̬
��4������������ɫ���ٸı��״̬
��5����������ƽ����Է����������ٸı��״̬��
A. ![]() B.
B. ![]() C.
C. ![]() D.
D. ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ŀǰ���緶Χ�ڵ���ԴΣ�����״���Ϊһ�ֽϺõĿ�������Դ�����й㷺��Ӧ��ǰ����
(1)��֪�ڳ��³�ѹ�·�Ӧ���Ȼ�ѧ����ʽ��
��CO(g)��2H2(g)![]() CH3OH(g)����H1����90 kJ��mol��1
CH3OH(g)����H1����90 kJ��mol��1
��CO(g)��H2O(g)![]() CO2(g)��H2(g)��H2����41 kJ��mol��1
CO2(g)��H2(g)��H2����41 kJ��mol��1
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��_______________________��
(2)���ݻ�ΪVL�������г���amol CO��2amol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ʱ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
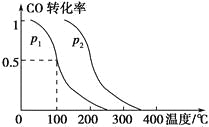
��p1________p2(����������������������������)��
���������������������£�������amol CO��2amol H2���ﵽ��ƽ��ʱ��CO��ת����________(����������������С����������������ͬ)��ƽ�ⳣ��________��
(3)��֪��T��ʱ��CO(g)��H2O(g)![]() CO2(g)��H2(g)��ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ(CO)��1 mol��L��1��cʼ(H2O)��1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________(�����Ѿ�������û����)�ﵽƽ�⣬ԭ����_________________________________����ʱ�̦���________����(����>������<��)��
CO2(g)��H2(g)��ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ(CO)��1 mol��L��1��cʼ(H2O)��1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________(�����Ѿ�������û����)�ﵽƽ�⣬ԭ����_________________________________����ʱ�̦���________����(����>������<��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2020������������״��������ϯ��ȫ���磬�й���ͳ��ҩ�����������������������翹�ߡ��������������䷽�к���ͳ��ҩ����������ԭ���ǽ�������Ҫ��������������Чҩ���ɷ�֮һ���ṹ��ʽ����ͼ��ʾ��������ԭ��������Ʋⲻ��������
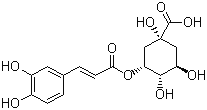
A.��������4������̼ԭ��B.������ˮҲ�������Ҵ�
C.1mol���ɺ�3mol NaOH��ӦD.��ͨ�����۷�Ӧ�γɸ߷���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Cu(s)��2H��(aq)===Cu2��(aq)��H2(g) ��H1
2H2O2(l)===2H2O(l)��O2(g) ��H2
2H2(g)��O2(g)===2H2O(l) ��H3
��ӦCu(s)��H2O2(l)��2H��(aq)===Cu2��(aq)��2H2O(l)����H��
A. ��H����H1��1/2��H2��1/2��H3 B. ��H����H1��1/2��H2��1/2��H3
C. ��H����H1��2��H2��2��H3 D. ��H��2��H1����H2����H3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com