
����Ŀ����֪�л���A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ�����ľۺϷ�Ӧ��Ʒ���ִ��ճ���������;�ܹ㡣һ�������£�A��ˮ��Ӧ����B��B�׳ƾƾ���B��ͭ�����ȴ��������¿��Ա���������ΪC�������������Ը��������Һ��Ӧ��ֱ������ΪD��B��Dһ�������·�Ӧ�IJ���E���������ϡ��ǹ�����ˮ�ͻ�ױƷ�е����ϡ�
�ش��������⣺
��1��A�����й����ŵ�������___��A�����������ӳɺ����F�ķ���ʽΪ___����F��Ϊͬϵ���������̼ԭ����Ϊ4���л�����һ�ȴ�����___�֡�
��2��д��B��D��Ӧ����E�Ļ�ѧ����ʽΪ___���÷�Ӧ�ķ�Ӧ����Ϊ___��
��3��B������ȼ�ϵ�أ�����NaOH���������Һ�������缫��Ӧ����ʽΪ___��
���𰸡�̼̼˫�� C2H6 4 CH3COOH+C2H5OH![]() CH3COOC2H5+H2O ������Ӧ����ȡ����Ӧ�� CH3CH2OH-12e-+16OH-=2CO
CH3COOC2H5+H2O ������Ӧ����ȡ����Ӧ�� CH3CH2OH-12e-+16OH-=2CO![]() +11H2O
+11H2O
��������
�л���A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪ��ϩ��һ�������£�A��ˮ��Ӧ����B��B�׳ƾƾ����Ҵ���B��ͭ�����ȴ��������¿��Ա���������ΪC��CΪ��ȩ�������Ը��������Һ��Ӧ��ֱ������ΪD��DΪ���ᣬB��Dһ�������·�Ӧ�IJ���EΪ����������
(1)A����Ϊ��ϩ�������ŵ�������̼̼˫������ϩ�����������ӳɺ����F�ķ���ʽΪC2H6�������黥Ϊͬϵ���������̼ԭ����Ϊ4���л���Ϊ��������춡�飬�����л���ĺ˴Ź���������2�֣����Զ����һ�ȴ�����4�֡�
(2)B��D��Ӧ����E�Ļ�ѧ����ʽΪCH3COOH+C2H5OH![]() CH3COOC2H5+H2O���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ����ȡ����Ӧ����
CH3COOC2H5+H2O���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ����ȡ����Ӧ����
(3)B������ȼ�ϵ�أ�����NaOH���������Һ����������������Ӧ�����������̼���������Ʒ�Ӧ����̼������ӣ��缫��Ӧ����ʽΪCH3CH2OH-12e-+16OH-=2CO![]() +11H2O��
+11H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2H4��һ�ָ�Ч���Ļ��ȼ�ϡ�8gN2H4(g)��ȫȼ�����ɵ�������̬ˮʱ���ų�133.5kJ�������������Ȼ�ѧ����ʽ����ȷ���ǣ� ��
A.![]() N2H4(g)��
N2H4(g)��![]() O2(g)=
O2(g)=![]() N2(g)��H2O(g) ��H����267kJ��mol��1
N2(g)��H2O(g) ��H����267kJ��mol��1
B.N2H4(g)��O2(g)=N2(g)��2H2O(g) ��H����534kJ��mol��1
C.N2H4(g)��O2(g)=N2(g)��2H2O(g) ��H����534kJ��mol��1
D.N2H4(g)��O2(g)=N2(g)��2H2O(l) ��H����133.5kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�к��зḻ��þ��Դ��ijͬѧ����˴�ģ�⺣ˮ���Ʊ�MgO��ʵ�鷽����

ģ�⺣ˮ�е�����Ũ��/mol��L��1 | Na�� | Mg2�� | Ca2�� | Cl�� | HCO3�� |
0.439 | 0.050 | 0.011 | 0.560 | 0.001 |
ע����Һ��ij�����ӵ�Ũ��С��1.0��10��5 mol��L��1������Ϊ�����Ӳ����ڣ�ʵ������У�������Һ������䡣Ksp[CaCO3]��4.96��10��9��Ksp[MgCO3]��6.82��10��6��Ksp[Ca(OH)2]��4.68��10��6��Ksp[Mg(OH)2]��5.61��10��12������˵����ȷ���ǣ� ��
A.������XΪCaCO3
B.��ҺM�д���Mg2����������Ca2��
C.��ҺN�д���Mg2����Ca2��
D.�����������Ϊ����4.2 g NaOH���壬������YΪCa(OH)2��Mg(OH)2�Ļ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����![]()
A.ͬʱ���з��Ӻ����ӵĵ������Һ��һ�������������Һ
B.pHΪ1�������У���![]() ��pHΪ3�������е�100��
��pHΪ3�������е�100��
C.����ʱ��![]() �������
�������![]() ��NaOH��Һ�������ϣ������Һ��
��NaOH��Һ�������ϣ������Һ��![]()
D.![]() ��Һ��
��Һ��![]() �����У���ˮ�������
�����У���ˮ�������![]() ���
���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
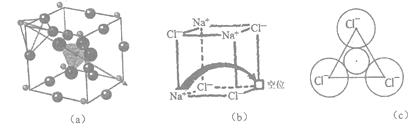
����Ŀ��[��ѧ-ѡ��3�����ʽṹ������]���������й㷺����;���о����֣�������������ṹΪ����(��Li+)�ṩ����Ǩ�Ƶ�ͨ�������С���ȱ�ݡ�������ʹ����е���DZ�������磺ͼ(a)��ʾ��﮳����ӵ���Li3SBF4��ͼ(b)��ʾ���С���ȱ�ݡ���NaCl��
������ѧ֪ʶ�ش��������⣺
(1)�ڱ仯��Cl+e-��Cl-�������У����õ�������ڻ�̬ Cl��________�ܼ����˹��̻�________ (����ա����ͷš�)������
(2)BF4-��B���ӻ���ʽΪ________________����ȵ�����Ϊ___________(��дһ��)������VSEPRģ����ͬ������l�Թµ��ӶԵ���Է���������С�ķ�����___________��
(3)ͼ(a)��ʾ������Li+λ��_____λ�ã�����������BF4-����F-���������������Խ��ͣ�ԭ����______________________________��
(4)ͼ(6)�У���ȱ�ݴ������Na+������__________(��ǡ����ǡ�) NaCl�ľ�������NaCl�����У�Na+�����Cl-�ѻ����ɵ�__________�����϶�С�
(5)������Ϊ���������С���ȱ�ݡ���NaCl���ﵼ��������������Na+Ǩ�Ƶ���һ��λ����ɡ�����Na+����һ����3��Cl-��ɵ���С�����δ���(��ͼc��ʾ)����֪��������a=564 pm��r(Na+)=116pm�� r(Cl-)=167 pm��ͨ�����������δ��װ뾶���жϸ���ʶ�Ƿ���ȷ��__________��(��֪��![]() ��1.414��
��1.414��![]() ��1.732)
��1.732)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺���Իش��������⣺
��1��Ni2+�����Ų��У���������������ߵ��ܼ�����Ϊ______��
��2������Ni����CO�γ������Ni��CO��4����CO��Ϊ�ȵ������һ�ַ���Ϊ______��д��ѧʽ����ͬ������CO��Ϊ�ȵ������һ������Ϊ______��
��3������ͪ뿣�![]() ���Ǽ���Ni2+�������Լ�������ͪ뿷�����Cԭ�ӹ���ӻ�����Ϊ______��2mol����ͪ뿷����������Ҽ�����ĿΪ______��
���Ǽ���Ni2+�������Լ�������ͪ뿷�����Cԭ�ӹ���ӻ�����Ϊ______��2mol����ͪ뿷����������Ҽ�����ĿΪ______��
��4������ͪ뿳���NI2+�γ�ͼA��ʾ������ͼB��������Ľ����
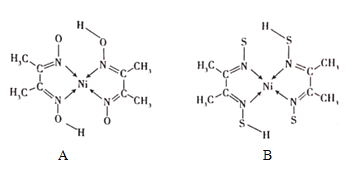
��A���ۡ��е����B��ԭ��Ϊ______��
��B���庬�л�ѧ��������Ϊ______����ѡ����ĸ����
A���Ҽ�B��������C����λ��D���м�
��5���˹��ϳɵ���������������ȱ�ݣ�ijȱ�������������ΪNi0.97O������NiԪ��ֻ��+2��+3���ּ�̬�����ּ�̬����������Ŀ֮��Ϊ______��
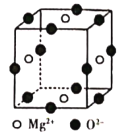
��6��Ni2+��Mg2+��O2-�γɾ���ľ����ṹ��ͼ��ʾ��Ni2+δ����������þ���Ļ�ѧʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ͻ�������ȶ���������ȼ����ʴ����ǿ���㷺Ӧ���ڽ�����ҵ��ȼ���ֻ���ij�ָ������Ͻ���Fe��Cr��Ni��C����Ԫ����ɡ�
(1)Fe��Cr��Ni�Ļ�̬ԭ�Ӻ������ռ�ݵ�����ܲ�Ϊ________(�����)��
(2)��̬Crԭ�Ӽ۲���ӵĵ����Ų�ʽΪ________����Crͬ�����һ�̬ԭ��������������ͬ��Ԫ�أ�λ�����ڱ���________����
(3)Fe3+��SCN-��Ӧ���ɺ�ɫ��K3Fe(SCN)6��K3Fe(SCN)6�ڲ�����������________��д��һ����SCN-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ________��
(4)��֪FeO��NiO�ľ���ṹ��ΪNaCl�ͣ���NiO���۵����FeO����������________��
(5)���ھ���ȱ�ݣ�Ni��ij��������Ļ�ѧʽΪNi0.88O����þ�����Ni2+��Ni3+���������Ϊ________��
(6)C60���ӽṹ���侧���ṹ��ͼ1��ͼ2��ʾ������C60����Ŀ�϶�в���K+���þ�����һ�������¾��г�����������ṹ��ͼ3��ʾ��

��C60������̼ԭ�ӵ��ӻ���ʽΪ________��
�ڸó����徧���У�K+�������C60Χ�ɵ�________��϶��________��϶�С�
����������A���������Ϊ(0��0��0)��B������Ϊ(1��1��1)������A���������K+���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NaOH��Һ�ζ��ڱ���������أ��ڱ����������H2A��Ka1=1.1��103 ��Ka2=3.9��106����Һ�������Һ����Ե��������仯������ͼ��ʾ������b��Ϊ��Ӧ�յ㡣���������������

A. �����Һ�ĵ�������������Ũ�Ⱥ������й�
B. Na+��A2�ĵ�������֮�ʹ���HA��
C. b��Ļ����ҺpH=7
D. c��Ļ����Һ�У�c(Na+)>c(K+)>c(OH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���һԪ��HA����Һ��KOH��Һ�������ϣ���������仯����ʵ���������±���
ʵ���� | ��ʼŨ��/��mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c(KOH) | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
�����жϲ���ȷ����
A��ʵ������Ӧ�����Һ�У�c(K��)>c(A��)>c(OH��)>c(H��)
B��ʵ������Ӧ�����Һ�У�c(OH��)=c(K��)��c(A��)=![]() mol/L
mol/L
C��ʵ������Ӧ�����Һ�У�c(A��)��c(HA)>0.1mol��L��1
D��ʵ������Ӧ�����Һ�У�c(K��)=c(A��)>c(OH��) =c(H��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com