
����Ŀ����֪NO��O2ת��ΪNO2�ķ�Ӧ�������£���2NO(g) ![]() N2O2(g)(��) ��H1<0��ƽ�ⳣ��K1,��N2O2(g)+O2(g)
N2O2(g)(��) ��H1<0��ƽ�ⳣ��K1,��N2O2(g)+O2(g) ![]() 2NO2(g)(��) ��H2<0��ƽ�ⳣ��K2������˵����ȷ���ǣ� ��
2NO2(g)(��) ��H2<0��ƽ�ⳣ��K2������˵����ȷ���ǣ� ��
A.2NO(g)+O2(g) ![]() 2NO2(g)�ġ�H=��H1+��H2
2NO2(g)�ġ�H=��H1+��H2
B.2NO(g)+O2(g) ![]() 2NO2(g)��ƽ�ⳣ��K= K1/K2
2NO2(g)��ƽ�ⳣ��K= K1/K2
C.��Ӧ�ٵ����ʴ�С����2NO(g)+O2(g) ![]() 2NO2(g)�ķ�Ӧ����
2NO2(g)�ķ�Ӧ����
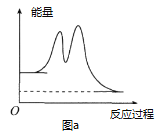
D.��Ӧ�����е������仯����ͼa��ʾ
���𰸡�A
��������
A.���ݸ�˹���ɷ�������+�ڼ��ɵõ���Ӧ��2NO(g)+O2(g)![]() 2NO2(g)���ʷ�Ӧ��Ϊ��H=��H1+��H2����Aѡ����ȷ��
2NO2(g)���ʷ�Ӧ��Ϊ��H=��H1+��H2����Aѡ����ȷ��
B. ��Ϊ��Ӧ2NO(g)+O2(g)![]() 2NO2(g)Ϊ��+�ڵĽ����������ƽ�ⳣ��K=K1K2����Bѡ�����
2NO2(g)Ϊ��+�ڵĽ����������ƽ�ⳣ��K=K1K2����Bѡ�����
C. ��Ӧ�������ʾ����ܷ�Ӧ���ʣ����Է�Ӧ�ڵ����ʴ�С����2NO(g)+O2(g)![]() 2NO2(g)�ķ�Ӧ���ʣ���Cѡ�����
2NO2(g)�ķ�Ӧ���ʣ���Cѡ�����
D.ͼa��ʾǰ��Ϊ���ȣ���������Ϣ�����ϣ���Dѡ�����
�ʴ�ѡA��


 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
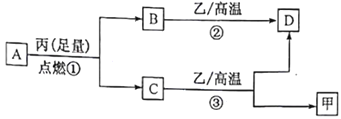
����Ŀ����֪X��Y��Z������ԭ��������������Ķ�����Ԫ�ء��ס��ҡ����ֱ�������Ԫ���γɵĵ��ʣ�A��B��C��D�ֱ���������Ԫ���е������γɵĻ������A��C�о�����10 �����ӡ�����֮��ת����ϵ����ͼ��ʾ������˵����ȷ����
A. ԭ�Ӱ뾶��Z>Y>X
B. X��Y�γɵĻ�����ֻ�����Լ�
C. Y�ж���ͬ�������壬�Ҿ����и��۵㡢�߷е㡢Ӳ�ȴ������
D. ��̬�⻯����ȶ��ԣ�A<C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧѧϰС���������ʵ�鷽�����ⶨij����NaCl��С�մ���Ʒ��![]() ��������������֪��
��������������֪��![]() ���ȷֽ��̼���ơ�������̼��ˮ��
���ȷֽ��̼���ơ�������̼��ˮ��
������һ����ȡһ��������Ʒ����������A�м��������غ���ȴ������ʣ��������������㡣
(1)����A������Ϊ___________��AӦ�÷���_________����ȴ�����ز�����Ŀ����________��
(2)��ʵ��ӳ����յ�A������ʼ�����һ��ƽ��ʵ��������Ҫ����____�Ρ���ƽ��ʵ���Ŀ����___________��
������������ȡһ����������Ʒ(��Ʒ������Ϊm0)������С�ձ��У�������ˮ�ܽ⣻��С�ձ��м�����������ʯ��ˮ�����ˣ�ϴ�ӡ������������������Ϊm1�����㡣(��֪̼���ʽ��Ϊ100��![]() ��ʽ��Ϊ84)��
��ʽ��Ϊ84)��
(3)��Ӧ����ʽΪ_____________������Ʒ��![]() �������������Ա�ʾΪ_________��
�������������Ա�ʾΪ_________��
������������Y�ι���ע��һ�������Ũ�ȵ�ϡ���Ტ����ȷ��ȡ��ag��Ʒ�����Ӻ�ע����(��ͼ)���ٽ�Y�ι���б��ʹ��Ʒ����Һ��ַ�Ӧ��
(4)a����̫��������___________�����ⶨ���ƫ�ߣ���ԭ�������______(ѡ�����)��
����Ʒδ��ȫ��Ӧ
���¶�δ�㶨����¼����
������![]() �ܽ�����Һ��
�ܽ�����Һ��
�������ģ���ȡһ��������Ʒ����ɴ�����Һ���ñ�����ζ���
(5)����������ȷ����_______(ѡ�����)��
�����ձ�����100mL������Һ
���÷�̪Ϊָʾ��
�۵���������Һ��ɫ���ܽ�����ɫ���ְ���Ӽ�Ϊ�ζ��յ�
��ʢ����ĵζ���δ��ϴ�����ʵ����ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��ȡ9.00g��������ˮ���ⶨ���۵�ˮ��ٷ��ʣ���������£�
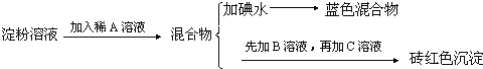
�Իش��������⣺
��1������������Լ�Ϊ��A_________��B_________��C_________��
��2������A��Һ��������B��Һ�Ƿ����_________����������_________��
��3��д������ˮ��ķ���ʽ_________��
��4��������1.44gש��ɫ����ʱ������ˮ������_________��[��֪��������Cu��OH��2��Ӧ�Ļ�ѧ����ʽΪ��CH2OH��CHOH��4CHO+2Cu��OH��2![]() CH2OH��CHOH��4COOH+Cu2O��+2H2O]��
CH2OH��CHOH��4COOH+Cu2O��+2H2O]��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ�����ֲ�����������彡������ʮ����Ҫ�����ã�Ҳ�㷺Ӧ�������Ͳ��ϵ��Ʊ���
(1)��̬��ԭ�ӵļ۵��ӹ������ʽ��_______________��������ͬ���������ڵ�����Ԫ�غ���ĵ�һ�������ɴ�С��˳��Ϊ___________��
(2)�������ʵĻ����ṹ��ԪΪ����ʮ���壬�����Է��س��ֶ��������Σ��������ʳ�Ϊ�����________��
(3)B�ļ��⻯��BH3����������ڣ����������γɽ��ȶ���B2H6�����������ӽ�ϡ�
��B2H6���ӽṹ��ͼ����Bԭ�ӵ��ӻ���ʽΪ________��

�ڰ�����(NH3BH3)����Ϊ�����DZ�������ʹ������֮һ�������д�����λ�����ṩ�µ��ӶԵijɼ�ԭ����______��д��һ���백���黥Ϊ�ȵ�����ķ���_____(�ѧʽ)��
(4)������(H3BO3)Ϊԭ�Ͽ��Ƶ����⻯��(NaBH4)�������л��ϳ��е���Ҫ��ԭ����BH![]() �ļ�����________�����幹��Ϊ___________��
�ļ�����________�����幹��Ϊ___________��
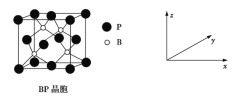
(5)����(BP)���ܸ߶ȹ�ע����ĥ���ϣ�����Ϊ��������ı����㣬��ṹ����ʯ���ƣ������ṹ��ͼ��ʾ��������z����ƽ���ͶӰͼ�У�Bԭ�ӹ��ɵļ�����״��_______����֪�����߳�Ϊ458 pm������������ܶ���____g��cm��3(��ʽ�����㣬���������λ��Ч���֣���֪4��583��96��07)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���õ�������������Na2SO4��ˮ��ԭ����ͼ��ʾ����Ĥ�м��Na����SO42-��ͨ�����ӽ���Ĥ�������˸��������Ӳ��ܽ����м���ҡ�

����������ȷ����(����)��
A.����·��ͨ��2 mol���ӵĵ���ʱ������2mol��H2����
B.�������õ�NaOH���������õ�H2SO4
C.������ӦΪ2H2O��4e��=O2��4H������������ҺpH����
D.ͨ����м���ҵ�SO42-������Ǩ�ƣ���������ҺpH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ڹ�ũҵ�������й㷺����;��
���������Ʊ������ܶࡣ
(1)�Ʊ�����һ��H2O2��Һ�������Ca(OH)2����Һ��Ӧ���Ʊ�CaO2��8H2O���仯ѧ����ʽΪ______________________________________________________________��
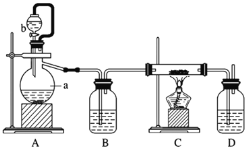
(2)�Ʊ������������÷�ӦCa(s)+O2![]() CaO2(s)���ڴ�����������ȡCaO2��ʵ����ģ��װ��ʾ��ͼ���£�
CaO2(s)���ڴ�����������ȡCaO2��ʵ����ģ��װ��ʾ��ͼ���£�
��ش��������⣺
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ________________������a������Ϊ ___________��
��װ��D��ʢ�е�Һ����Ũ���ᣬ������һ��_________������_____________��
��ˮ�������г���ˮ�м�һ����CaO2��8H2O������������DO����ˮ����������DO������ÿ��ˮ���ܽ���������������ʾ����ⶨ���輰ԭ��Ϊ��
a�������������£�O2��Mn2+����ΪMnO(OH)2��2Mn2++O2+4OH=2MnO(OH)2����
b�������������£�MnO(OH)2��I����ΪI2��MnO(OH)2+2I+4H+=Mn2++I2+3H2O��
c���ζ�����Na2S2O3����Һ�ζ����ɵ�I2��2S2O32-+I2=S4O62-+2I��
ijͬѧ��ˮ�м�һ����CaO2��8H2O��ȡ��ˮ��100.00mL�������������ⶨˮ����������DO��������0.0100mol��L1 Na2S2O3����Һ13.50mL��
(1)�ζ�������ʹ�õ�ָʾ����_______________________________��
(2)��ˮ���е��ܽ�������DO��Ϊ__________________mg��L1��
(3)����b�м���������Һ��Ӧ������ҺpH���ͣ��ζ�ʱ��������Ե���д������������ԭ��________________________�������ӷ���ʽ��ʾ������д��2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�AC2�ǷǼ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2:1����������������硣��������������ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ_________��
(2)B���⻯��ķ������幹����_____��������ԭ�Ӳ�ȡ_______�ӻ���
(3)д��������AC2�ĵ���ʽΪ_______��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ______��
(4)E�ĺ�������Ų�ʽ��______��ECl3�γɵ������Ļ�ѧʽΪ_____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
A.22.4L��ϩ��C-H����Ϊ4NA
B.1.5g���е�����Ϊ1.0NA
C.��״���£�22.4L���к��е�̼ԭ����Ϊ6.0NA
D.�����£�21.0g��ϩ�Ͷ�ϩ�Ļ�������к��е�̼ԭ����ĿΪ1.5NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com