
����Ŀ��̼���γɻ�������������Ԫ�أ��䵥�ʼ��������ж��ص����ʺ���;����ش��������⡣
��1��̼ԭ�Ӻ��������_____�ֲ�ͬ���˶�״̬��̼ԭ�ӵļ۵������γ�sp3�ӻ�����������ʽΪ_____��
��2��д��һ��CO32-�ĵȵ��������Ļ�ѧʽ_______����ռ乹��Ϊ_______��
��3���л���M( )��һ������������N(
)��һ������������N( )��
)��
�ٷе㣺M_____N ����������������С��������
��M��̼ԭ���ӻ�����Ϊ_____����ͬ�ӻ����͵�̼ԭ����֮��Ϊ_____��
���л���N�г���ԭ��֮�������ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ_____��
��4����֪CaCO3���ȷֽ��¶�Ϊ900�棬SrCO3���ȷֽ��¶�Ϊ1172�����Դ�ԭ�ӽṹ�ĽǶȽ���CaCO3���ȷֽ��¶ȵ���SrCO3��ԭ��_____________��
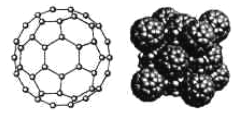
��5��̼��һ��ͬ��������C60����������ϩ����һ�ָ߶ȶѳɵ���̼���ӡ������飨����ʽ��C8H8��![]() ���DZ�C60Լ��20��ϳɳ���һ�ֶԳ���������ӣ�������Ѻϳɳ�һ����������C60�ĸ����ͷ��Ӿ��壬�þ���ľ����ṹ����ͼ��ʾ����������������ԭC60����ķ��Ӽ��϶�С���ø����ͷ��Ӿ��������ö��ߵķ���ʽ�ɱ�ʾΪ______________��
���DZ�C60Լ��20��ϳɳ���һ�ֶԳ���������ӣ�������Ѻϳɳ�һ����������C60�ĸ����ͷ��Ӿ��壬�þ���ľ����ṹ����ͼ��ʾ����������������ԭC60����ķ��Ӽ��϶�С���ø����ͷ��Ӿ��������ö��ߵķ���ʽ�ɱ�ʾΪ______________��
��6��ʯī��̼��һ��ͬ�������壬����һ�־����ṹ�Ͳ��־�����������ͼ��
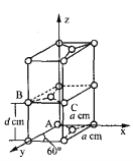
��ԭ����������������Ǿ�����ԭ�Ӽ�����λ�á�ʯī������̼ԭ��A��B����������ֱ�Ϊ��A(0��0��0)��B(0��1��1/2)����Cԭ�ӵ��������Ϊ_______________��
�ھ��������������������Ĵ�С����״����֪ʯī�����ױ߳�Ϊacm ������Ϊdcm�������ӵ�������ֵΪNA����ʯī���ܶ�Ϊ_____g��cm-3��д������ʽ���ɣ���
���𰸡� 6 ![]() SO3�� NO3- ��SiO32- ƽ�������� С�� sp2��sp3 7��2 N > O > C Ca2+�����Ӱ뾶С��Sr2+��Ca2+�����̼��������е������ӣ�ʹ̼������Ӹ��ֽ�Ϊ������̼ C8H8��C60 ��1��1��1/2��
SO3�� NO3- ��SiO32- ƽ�������� С�� sp2��sp3 7��2 N > O > C Ca2+�����Ӱ뾶С��Sr2+��Ca2+�����̼��������е������ӣ�ʹ̼������Ӹ��ֽ�Ϊ������̼ C8H8��C60 ��1��1��1/2�� ![]() g��cm-3
g��cm-3
��������(1).��Ϊû���˶�״̬��ͬ�ĵ��ӣ�����Cԭ�Ӻ�����6�в�ͬ�˶�״̬�ĵ��ӣ�̼ԭ�ӵ�2S���������2P��������ӻ���ͬʱ�����������ȵ���ռ��һ�������������������ʽΪ��![]() ��
��
(2). SO3��CO32-��Ϊ�ȵ����壬Sԭ����sp2�ӻ�����ɼ�, SO3����Ϊƽ���������η��ӣ�
(3). �ٶԽṹ���Ƶ��л���������Խ��е��Խ�ߣ����Էе㣺MС��N������ΪM�к���C-C�������Լ�C=O������M��C���ӻ���ʽΪsp2��sp3�ӻ��������ӻ���ʽ��̼ԭ�ӱ���Ϊ����6+1��:2=7:2 ������N��O��C�У���һ�����ܵ�˳��ΪN > O > C��
(4). ��SrCO3��CaCO3��Ϊ���Ӿ��壬SrCO3��CaCO3�������������ȣ�����Ca2+�뾶С��Sr2+�뾶������CaO�����ܴ���SrO�����ܣ�����Ca2+��Sr2+������̼��������е������ӽ�ϣ�ʹ̼������ӷֽ�ΪCO2��
(5).������C60��ĿΪ8*1/8+6*1/2=4����������������ԭC60����ķ��Ӽ��϶�У���������������ĿΪ4�������ʽΪC8H8��C60��
(6). ����B������ɵã�a=1��d=1/2������C������Ϊ��1��1��1/2��������ʯī�ľ����ṹ�þ�����Cԭ�ӵ�����Ϊ1+2��1/2+8��1/8+4��1/4=4,�������Ϊ2d��a��a��sin60��=![]() , ��ʯī���ܶ�Ϊ
, ��ʯī���ܶ�Ϊ![]() g��cm-3��
g��cm-3��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ��ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O��
(1)��������װ��A��ȡNa2S2O3��

��д������װ��A�з�����Ӧ�Ļ�ѧ����ʽ_______________________��
��װ��B�������Ǽ���װ����SO2������Ч�ʣ�B���Լ���_____________������SO2����Ч�ʵ͵�ʵ��������B ����Һ___________________ ��
��Ϊ��ʹSO2������������ȫ�����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��___________(д��һ������)��
(2)�ӷ�ӦҺ�л��Na2S2O3��5H2O����ķ�����_____________����ѹ���ˣ�ϴ�Ӹ��
(3)ʵ���Ƶõ�Na2S2O3��5H2O��Ʒ�п��ܺ���Na2SO3��Na2SO4�����ʡ������ʵ����Na2S2O3��5H2O��Ʒ���Ƿ����Na2SO4���ʣ���Ҫ˵��ʵ���������ͽ��ۣ�__________________________��
(4)��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡagKIO3(��Է���������214)���������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
����������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����ΪVmL
��c(Na2S2O3)=________mol��L-1 (�г���ʽ����)��
(��֪��IO3-+5I-+6H+=3I2+3H2O��2S2O32-+I2= S4O62-+2I-)
���ڵζ�����������ʵ����������ʵ����ƫ�͵���_______��
A.�ζ���δ��Na2S2O3��Һ��ϴ
B.�ζ��յ�ʱ���Ӷ���
C.��ƿ������ˮ��ϴ��δ���и��ﴦ��
D.�ζ��ܼ��촦�ζ�ǰ�����ݣ������յ㷢��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������м�����ϡH2SO4��Ӧ��������NaOH��Һ��Ӧ���Ǣ�Al2O3��NaHSO4��NaHCO3��Al(OH)3��Na2CO3�� ��
A.�٢ۢ�B.�ڢۢ�C.�ڢܢ�D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������������У���ȷ���ǣ� ��
A.���Լ�ƿ��ȡ�����κ�ҩƷ������ʣ����ٷŻ�ԭ�Լ�ƿ
B.��ϡ����ϴ��ʢ�Ź�ʯ��ˮ���Լ�ƿ
C.�ƾ��Ʋ����������ʱ����ˮ����
D.����һ��������ͬ����ֽ����ƽ�������ϣ���NaOH�����������ֽ�ϳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֤���Ҵ��ķ������ΪCH3CH2OH��������CH3OCH3�����ֵ������� (����)
A. 1 mol�Ҵ��������Ʒ�Ӧ�ų�0.5 mol����
B. 1 mol�Ҵ���ȫȼ����Ҫ3 mol����
C. 1 mol����2 mol������̼��3 molˮ
D. 4.6 g�Ҵ��ڱ�״���µ����Ϊ2.24 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ǧ��������Ǧ�������ᣨHBF4�������һ�ֵ������������ĵ�أ������ھ��º�����ҵ�����ܷ�Ӧ����ʽΪPb+PbO2+4HBF4![]() 2Pb��BF4��2+2H2O[��֪��HBF4��Pb��BF4��2����������ˮ��ǿ�����]������˵���в���ȷ���ǣ� ��
2Pb��BF4��2+2H2O[��֪��HBF4��Pb��BF4��2����������ˮ��ǿ�����]������˵���в���ȷ���ǣ� ��
A. �ŵ�ʱ����Һ�е�BF4-���ƶ�
B. �ŵ�ʱ��ת��1mol����ʱ�������ٵ�����Ϊ119.5g
C. ���ʱ������������Һ�����Լ���
D. ���ʱ�������ĵ缫��ӦʽΪPb2++2e-�TPb
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ƿ�չ�е�����Դ���������ð�������Ʊ��������Ӧ���������ڡ��ش��������⣺
��1����������ȣ�������Ϊȼ���������ŵ�����������ֱ��ȼ�յ�����ת����Զ����ȼ�ϵ�أ�д����������ȼ�ϵ�صĸ�����Ӧʽ��________________________________��
��2�������������Ʊ�H2O2��
��֪��H2(g)��A(l)===B(l)����H1
O2(g)��B(l)===A(l)��H2O2(l)����H2
����A��BΪ�л������Ӧ��Ϊ�Է���Ӧ����H2(g)��O2(g)===H2O2(l)����H________0(����>����<����������)��
��3���ں��º��ݵ��ܱ������У�ij���ⷴӦ��MHx(s)��yH2(g) ![]() MHx��2y(s)����H<0�ﵽ��ѧƽ�⡣�����й�������ȷ����________��
MHx��2y(s)����H<0�ﵽ��ѧƽ�⡣�����й�������ȷ����________��
a������������ѹǿ���ֲ��� b������y mol H2ֻ��1 mol MHx
c�������£��÷�Ӧ��ƽ�ⳣ������ d������������ͨ��������������v(����)>v(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ������п��ɰ����Ҫ��ZnO��ZnFe2O4������������CaO��FeO��CuO��NiO���������ȡ����п��������ͼ��ʾ���ش��������⣺
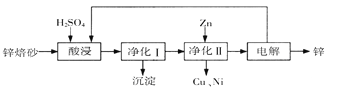
��1�����ʱZnFe2O4�����������Σ��÷�Ӧ�Ļ�ѧ����ʽΪ
��2������I������Ϊ��������һ���ǽ���Һ��������Fe2���������ڶ����ǿ�����ҺpH��ʹFe3��תΪFe(OH)3������
��д������������H2O2��Fe2����Ӧ�����ӷ���ʽ
��250Cʱ��pH=3����Һ�У�c (Fe3��)�� mol/L����֪25����![]() ��
��
������I���ɵij����л�������Һ�е��������ʣ���Һ�е��������ʱ���ͬ������ԭ���� ��
��3����û�о���II���������п���Ʊ�������Ӱ���� ��
��4���������п���ѭ�����õ����ʳ�п��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ƿ�������������ʵ���Ũ�ȵ���Һ�Ķ������������ϱ��У����¶� ��Ũ�� ������ ��ѹǿ�ݿ̶��� ����ʽ���ʽ�������еģ� ��
A.�ڢܢ�
B.�ۢݢ�
C.�٢ڢ�
D.�٢ۢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com