
����Ŀ����.��1��������Һ�õ�������ע��Һ�������ǣ���ѧʽΪC6H12O6����ˮ��Һ�����ǩ�ϵIJ�����������ͼ��ʾ�����ñ�ǩ���ṩ����Ϣ�������ע��Һ�������ǵ����ʵ���Ũ��Ϊ____________ ��������λ��Ч���֣���

��2������˵�ѪҺ�������ǣ����Ѫ�ǣ��ĺ������ο�ָ�곣�����ּ�����λ��ʾ������mmol/L���͡�mg/dL��(1L=10dL)���ԡ�mmol/L����ʾʱ���˵�Ѫ������ֵ��3.61~6.11 mmol/L ֮�䡣��ij�˵�Ѫ�Ǽ����Ϊ92mg/dL������Ѫ��������_________ �������������������
��.����0.270kg��������Ϊ10%��CuCl2��Һ������
��1����Һ��CuCl2�����ʵ���Ϊ___________��
��2��Ҫ����Һ�е�Cl-��ȫ�����������1.0 mol/L��AgNO3��Һ_______mL��
��3��Ҫ����Һ�е�ͭ��ȫ�û������������Fe ������Ϊ__________��
���𰸡�0.28mol/L ���� 0.2mol 400 11.2g
��������
��.��1��C6H12O6��Ħ������Ϊ180g/mol��25g�����ǵ����ʵ���Ϊ25/180mol����Һ���Ϊ500 mL����ע��Һ�������ǵ����ʵ���Ũ��Ϊ(25/180)/0.5��0.28mol/L������������������ǣ�0.28mol/L��
��2��92mg/dL=(92��10-3)/180mol/dL=10��[(92��10-3)/180]mol/L=103��10��[(92��10-3)/180] mmol/L=5.11mmol/L, �˵�Ѫ������ֵ��3.61~6.11 mmol/L ֮��,���Ը���Ѫ������������������������ǣ�������
��.��1��0.270kg��������Ϊ10%��CuCl2��Һ��CuCl2�����ʵ���Ϊ��0.270��103��10%��/135=0.2mol ������������������ǣ�0.2mol��
��2�����ݣ�1����֪��0.2molCuCl2�к��������ӵ���Ϊ0.4mol�����ݷ�ӦAg++Cl-=AgCl����Ӧ��֪�����������ӵ���Ϊ0.4mol�����ݹ�ʽ��n=c��V��֪��1.0��V=0.4��V=0.4L=400 mL������������������ǣ�400 ��
��3�����ݷ�Ӧ��Fe+Cu2+=Fe2++Cu��֪��0.2molCuCl2�к���Cu2+0.2mol������������Ϊ0.2mol������Ϊ0.2��56=11.2g������������������ǣ�11.2g��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ����ȷ����
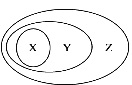
X | Y | Z | |
A | ˮ | ���� | ������ |
B | �ǽ��������� | ���������� | ������ |
C | ����� | ������ | ������ |
D | ���Ϸ�Ӧ | ������ԭ��Ӧ | ��ѧ��Ӧ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ����2molCO��5molH2�Ļ����������ݻ�Ϊ2L���ܱ����������ڴ����������·�����Ӧ:CO(g)+2H2(g)![]() CH3OH(g)��
CH3OH(g)��
��1������5min��Ӧ�ﵽƽ�⣬��ʱת�Ƶ���6mol���÷�Ӧ��ƽ�ⳣ��Ϊ_______��V(H2)=_____mol/(L��min)��������������䣬�ٳ���2mulCO��1.5mol CH3OH����ʱv(��)___ v(��)(����>����<������=��)��
��2���������������������£�������2molCO��5molH2���ﵽ��ƽ��ʱ��CO��ת����____(��������������С������������)��
��3�����в���˵���÷�Ӧ�Ѵﵽƽ��״̬����__________
a.CH3OH���������� b.��������ƽ����Է����������ٸı�
c.V��(CO)=2V��(H2) d.���������ܶȲ��ٷ����ı�
��4����һ��ѹǿ�£��ݻ�ΪVL����������amolCO��2amolH2,�ڴ��������·�Ӧ���ɼ״�,ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
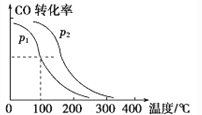
��p1_____p2(������������С������������������ͬ)����H_____0���÷�Ӧ��_______(��������������������)�����Է����С�
��5����ʹ�÷�Ӧ�ķ�Ӧ��������,��ƽ��������Ӧ�����ƶ�����_____��
a.��ʱ�����CH3OH���� b.�ʵ������¶�
c.����H2��Ũ�� d.ѡ���Ч����
��6�������ĸ�ѡ������λѧ����ѧϰ��ѧ��Ӧ�����뻯ѧ��Ӧ���Ժ�,��ϵ��������ʵ���������Ŀ���������Ϊ����ȷ����_______��
a.��ѧ��Ӧ�������ۿ�ָ��������һ��ʱ���ڿ����Ʒ
b.��Ч��ײ���ۿ�ָ���������ԭ�ϵ�ת����
c.��������ԭ����ָ������ʹ������ԭ�϶����Ʒ
d.��ȷ���û�ѧ��Ӧ���ʺͻ�ѧ��Ӧ�ȶ�����������������ۺϾ���Ч��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ʻ�����COS������Ϊһ����ʳѬ�������ܷ�ֹijЩ���桢�߳�������Σ�����ں����ܱ������У���CO��H2S��ϼ��Ȳ��ﵽ����ƽ�⣺CO(g)+H2S(g)![]() COS(g)+H2(g) K=1����ӦǰCO���ʵ���Ϊ1mol��ƽ���CO���ʵ���Ϊ0.5mol������˵����ȷ������ ��
COS(g)+H2(g) K=1����ӦǰCO���ʵ���Ϊ1mol��ƽ���CO���ʵ���Ϊ0.5mol������˵����ȷ������ ��
A. �ʻ���ĵ���ʽΪ�� :![]() :
:![]() :
:![]() :
:
B. ͨ��CO������Ӧ����������
C. �ټ���0.1molH2S��0.1molH2��ƽ�ⲻ�ƶ�
D. ��ӦǰH2S���ʵ���Ϊ0.25mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ó���������ˮ��ϴ���ú��ֺ���ɫ����ߣ��ڴ˱仯�����в������Ļ�ѧ��Ӧ��
A. 4Fe(OH)2��2H2O��O2===4Fe(OH)3��
B. 2Fe��2H2O��O2===2Fe(OH)2��
C. 2H2O��O2��4e��===4OH��
D. Fe��3e��===Fe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Fe��Cu��ϡH2SO4��ɵ�ԭ����С����������������
A.����������������������ͭ��B.��Һ��Ϊdz��ɫ
C.��Һ�����Լ���D.��������Ƭ�ϲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ������ش��й����⣺
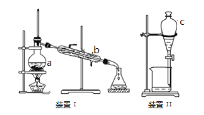
��1��д��װ��I���й����������ƣ�a ________________��b__________________����װ�÷��������ԭ���Ǹ��ݻ��������______________��ͬ���з���ġ�
��2��װ��II������C ������Ϊ_______________��ʹ��Cǰ������еIJ����� _______________ΪʹC��Һ��˳�����£�����ǰӦ���еIJ����� _________________________��
��3����������36.5%��Ũ���ᣨ��=1.19g/cm3������250mL 0.5mol/L��ϡ���� �IJ������밴Ҫ����գ�
������������Һ����IJ�������������������Ͳ���ձ������� _____________________����Ҫ��ȡŨ��������Ϊ ____________mL��
�����в�����,�����������ҺŨ��ƫ�͵���______(����ĸ)��
a������ʱ�����ӿ̶��� b��û��ϴ���ձ��Ͳ�����
c��ϴ�Ӻ������ƿ�в�����������ˮ��d����Ͳ��ȡ����ǰû����Ũ������ϴ��
�۾��ⶨ��������Һ��Ũ��ƫС���������ʵ����̲����������ܵ�ԭ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ʣ��������������塡��KHSO4����HNO3 ��ϡ���� �ݶ�����̼���塡��ͭ����̼���Ʒ�ĩ�������Ǿ��塡�������Ȼ��ơ���CuSO4��5H2O���塣���������գ�
��1������״̬�¿ɵ������____________________________________��
��2�����ڵ���ʵ���__________________________________________��
��3�����ڷǵ���ʵ���____________________________________��
��4������ˮ��Һ�еĵ��뷽��ʽΪ_____________________�����������Һ�з�Ӧʹ��Һ�����Ե����ӷ���ʽΪ_______________________��
��5��������Է������·�Ӧ��Cu��4HNO3(Ũ)��Cu(NO3)2��2NO2����2H2O���ش��������⣺
I����ԭ������________��
II.����2 mol HNO3�μӷ�Ӧʱ�������������ʵ�����Ϊ________g��
III.��˫���ű�ʾ�÷�Ӧ����ת�Ƶķ������Ŀ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʣ�������Na��Ӧ�ų�H2�������H2����������˳����ȷ���ǣ� ��
��C2H5OH ��ϡ���� ��H2O
A.��>��>��B.��>��>��
C.��>��>��D.��>��>��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com