
����Ŀ������ȫ����������������Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����е������̼���������Ⱦ����һ����Ҫ����Ĺ�����
��������������о�
��1��һ�������£���2mol NO��2mol O2���ں����ܱ������з�����Ӧ��2NO(g)+O2(g) ![]() 2NO2(g)������״̬��˵���÷�Ӧ�ﵽ��ѧƽ�������_______��
2NO2(g)������״̬��˵���÷�Ӧ�ﵽ��ѧƽ�������_______��
A�����������ܶȱ��ֲ���
B��NO��ת���ʱ��ֲ���
C��NO��O2�����ʵ���֮�ȱ��ֲ���
D��O2���������ʺ�NO2�������������
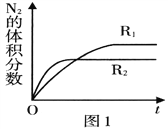
��2����֪��Ӧ. 2NO(g) ![]() N2(g)+O2(g) ��H<O���ڲ�ͬ����ʱN2�����������ʱ��(t)�ı仯��ͼ1��ʾ������ͼ������ж�����R1��R2��Ӧ�����з�Ӧ�����в�ͬ����______ (����ĸ���)��
N2(g)+O2(g) ��H<O���ڲ�ͬ����ʱN2�����������ʱ��(t)�ı仯��ͼ1��ʾ������ͼ������ж�����R1��R2��Ӧ�����з�Ӧ�����в�ͬ����______ (����ĸ���)��
A��ѹǿ B���¶� C������
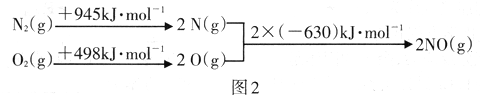
����ͼ2�е������仯���ݣ����㷴Ӧ2NO(g) ![]() N2(g)+O2(g)����H=__________
N2(g)+O2(g)����H=__________
����̼�������о�
��3��CO��H2��һ�������¿��Ժϳɼ״���CO(g)+2H2(g) ![]() CH3OH(g) ��H<O ���������Ϊ1L�ĺ����ܱ�������ͼ3������ͨ��1mol CO��2mol H2���ⶨ��ͬʱ�䡢��ͬ�¶�(T)��������CO�����ʵ��������±���
CH3OH(g) ��H<O ���������Ϊ1L�ĺ����ܱ�������ͼ3������ͨ��1mol CO��2mol H2���ⶨ��ͬʱ�䡢��ͬ�¶�(T)��������CO�����ʵ��������±���

��ش���
��T1_____ T2������>������<������=������������____________________����֪T2��ʱ����20minʱ������ѹǿ���ٸı䣬��ʱH2��ת����Ϊ_________�����¶��µĻ�ѧƽ�ⳣ��Ϊ________ ��
������1mol CO��2mol H2ͨ��ԭ���Ϊ1L�ĺ�ѹ�ܱ�������ͼ3�����У���T2���´ﵽƽ�⣬��ʱ��Ӧ��ƽ�ⳣ��Ϊ_________;������������ͨ��l mol CH3OH(g)�����´ﵽƽ���CH3OH(g)����ϵ�еİٷֺ���_________(���������������С������������)��
��4��һ��������Ҳ����NaOH��Һ��CO��Ӧ���ɼ����ƣ���һ����Ӧ���ɼ���������CO��Ⱦ�������½�a mol��COͨ��2 L bmol/L NaOH ��Һ�У�ǡ����ȫ��Ӧ���ɼ����ƺͺ���������Ļ����Һ(������Һ�������)�������Һ��c(Na+)=c(HCOO-)����û����Һ�м���ĵ���ƽ�ⳣ�� Ka=____________ (�ú�a��b�Ĵ���ʽ��ʾ)��
���𰸡� BC B ��183kJ.mol-1 �� �¶�Խ�ߣ���Ӧ����Խ�졣������������ͬʱ��T2ʱ�ķ�Ӧ���ʱ�T1�ķ�Ӧ���ʿ� 50%��0.5 1 1 ���� ��2b��10��7��/��a��2b��
����������.(1)A����Ӧ����������Ϊ���壬�����������䣬���������ܶ�ʼ�ձ��ֲ��䣬��A����B��NO��ת���ʱ��ֲ��䣬˵�����淴Ӧ������ͬ��B��ȷ��C��NO��O2��Ͷ�ϱȲ����ڼ�����֮�ȣ�NO��O2�����ʵ���֮�ȱ��ֲ��䣬˵�������ʵ���Ũ�ȱ��ֲ��䣬��C��ȷ��D��O2���������ʺ�NO2�������������ʱ�����淴Ӧ���ʲ����ȣ���D����ѡBC��(2)A��R2��Ӧѹǿ�ߣ���ѹ��ƽ�������ƶ���N2���������Ӧ��Щ����A����B��R2��Ӧ���¶ȸߣ����ȷ�Ӧ�����º�ƽ�������ƶ���N2���������ӦСЩ����B��ȷ��C��������ƽ��û��Ӱ�죬��C����ѡB������ͼ2�е������仯���ݣ����㷴Ӧ2NO(g) ![]() N2(g)+O2(g) �Ħ�H=��������������kJ��mol-1 ����������kJ��mol-1 ��������kJ��mol-1���� -183kJ��mol-1
N2(g)+O2(g) �Ħ�H=��������������kJ��mol-1 ����������kJ��mol-1 ��������kJ��mol-1���� -183kJ��mol-1
��.��������T1��T2���¶�Խ�ߣ���Ӧ����Խ�졣������������ͬʱ��T2ʱ�ķ�Ӧ���ʱ�T1ʱ�ķ�Ӧ���ʿ죻T2��ʱ����20minʱ������ѹǿ���ٸı䣬
CO(g)+2H2(g) ![]() CH3OH(g)
CH3OH(g)
nʼ/mol 1 2 0
n��/mol 0.5 1 0.5
nƽ/mol 0.5 1 0.5
cƽ/mol/L 0.5 1 0.5
H2��ת����Ϊ=1mol/2mol��100%=50%,���¶��µĻ�ѧƽ�ⳣ��Ϊ=![]() �����¶Ȳ��䣬ƽ�ⳣ�����䣬��Ϊ1��������������ͨ��1molCH3OH(g)�����´ﵽƽ���CH3OH(g)����ϵ�еİٷֺ������䣬�൱����������Чƽ�⡣
�����¶Ȳ��䣬ƽ�ⳣ�����䣬��Ϊ1��������������ͨ��1molCH3OH(g)�����´ﵽƽ���CH3OH(g)����ϵ�еİٷֺ������䣬�൱����������Чƽ�⡣
(4) �������غ�c(HCOOH)+c��HCOO�D ��= ![]() ��c(Na+)=c(HCOO-)=bmol/L��c(HCOOH)=
��c(Na+)=c(HCOO-)=bmol/L��c(HCOOH)= ![]() ���ɵ���غ�c(H�� )=10-7mol/L,K=
���ɵ���غ�c(H�� )=10-7mol/L,K= ![]() ��10-7��
��10-7��![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)0.5 mol H2O������Ϊ____________�����к���_________��ˮ���ӣ���ԭ�ӵ����ʵ���Ϊ__________��
(2)������ͬ��H2��NH3��SO2��O3���������У����з�����Ŀ���ٵ���________������ͬ�¶Ⱥ���ͬѹǿ�����£����������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ�����⡣���պϸ�װ�õĵ��ʱ���۲쵽�����Ƶ�ָ�뷢����ƫת����ش��������⣺

��1���׳���ͨ��CH4�缫�ĵ缫��ӦΪ___________________________________��
��2���ҳ���A��ʯī���缫������Ϊ________����������������������������������������� �ҳ����ܷ�ӦʽΪ____________________________________ ��
��3�����ҳ���B����������5.40gʱ���׳�������������O2�����Ϊ______mL����״������������______���C����D����������_______gͭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�ΪԪ�����ڱ������ڵ�һ���֣������й�A��B��C��D����Ԫ�ص�������ȷ����
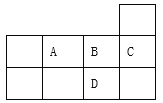
A. ԭ�Ӱ뾶��СΪ��B>A>C
B. �˵������D > C>B >A
C. A��D�γɵĻ�����������Ӽ�
D. ���ɵ��⻯����ȶ���Ϊ�� D >A>B
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����λ���������������ɺͽṹ����ɫɫ���ḻ��ʡ���ָ������� [Cu(H2O)4](OH)2���������ӡ����塢�������ӵĵ��������λ��
A��Cu2����H2O����2��4 B��Cu����H2O����1��4
C��Cu2����OH������2��2 D��Cu2����H2O����2��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʣ���O2��O3 ��H2��D2��T2 ��12 C��14 C
��CH3CH2CH2CH2CH3��(CH3)2CHCH2CH3 �ݹ����ʮ���� ��CH3(CH2)5CH3��CH3CH2CH(CH3)C2H5
��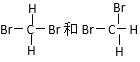
A����Ϊͬλ�ص���_________��B����Ϊͬ���칹�����________��
C����Ϊͬ�����������________��D��ͬһ�����ʵ���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������ۻ�������ʱ,���˷����������������ͬ�����͵���
A��SiO2�ɱ����ۻ� B�������ƺ��Ƶ��ۻ�
C�������;�������ۻ� D����ɱ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4NH3��������5O2������![]() 4NO��������6H2O��������10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������
4NO��������6H2O��������10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������ ![]() (X)(��Ӧ����������ʻ�������������)�ɱ�ʾΪ
(X)(��Ӧ����������ʻ�������������)�ɱ�ʾΪ
A��![]() (NH3) = 0.010 mol/��L��s��
(NH3) = 0.010 mol/��L��s��
B��![]() (O2) = 0.0010 mol/��L��s��
(O2) = 0.0010 mol/��L��s��
C��![]() (NO) = 0.0010 mol/��L��s��
(NO) = 0.0010 mol/��L��s��
D��![]() (H2O) = 0.045 mol/��L��s��
(H2O) = 0.045 mol/��L��s��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�顣
��.��1�����ơ��ء�þ������1 mol�ֱ�Ͷ�뵽������0.1 mol��L��1�������У�д�������ᷴӦ�����Ľ���������Ӧ�����ӷ���ʽ__________��
��2����NaOH��Һ��NH4Cl��Һ�������NH3��H2O���Ӷ���֤NaOH�ļ��Դ���NH3��H2O���̶�������֤Na�Ľ����Դ���N������Ϊ������Ƿ����___________����˵�����ɣ�________________��
��.������ͼװ�ÿ�����֤�ǽ����Եı仯���ɡ�

��3��ʵ����������ҩƷNa2S��KMnO4��Ũ���ᡢMnO2����ѡ�����ҩƷ���ʵ����֤�ȵķǽ����Դ�����װ��B����װҩƷΪ_____________��װ��C�е�ʵ������Ϊ�е���ɫ�������ɣ����ӷ���ʽΪ_________________________��
��4����Ҫ֤���ǽ����ԣ�C>Si����A�м�________��B�м�Na2CO3��C�м�Na2SiO3���۲쵽C�е�ʵ������Ϊ�а�ɫ���������ɡ����ӷ���ʽΪ____________________��������ͨ�����ǹ����ģ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com