
����Ŀ��̼���仯����㷺��������Ȼ���С��ش��������⣺
(1)��̬̼ԭ�Ӻ�����________��������ͬ�ĵ��ӣ� ���������˶�״̬��_____ �����ؾ�����
(2)CH4���Ӽ䲻���γ������ ��Ҫԭ����CH4 �����е�̼ԭ�Ӳ����¶Ե��ӡ�_____ �� _____________��
(3)̼��ķ��ǻ����ĸ���������ķ��ǻ����ĸ�������1, �ӽṹ�Ϸ��������ǵ�ǿ�� �������Ϊ��ǿ�ᡣȻ����ʵ�϶�����̼ˮ��Һ������ȴ������ԭ����__________��
(4)�Ҷ���(H2NCH2CH2NH2)�� һ���л������ N ԭ�ӵ��ӻ��������Ϊ______���Ҷ���ͨ����λ������Cu2+ �γ��ȶ��Ļ�״�����ӣ���ṹ�ɱ�ʾΪ__________��
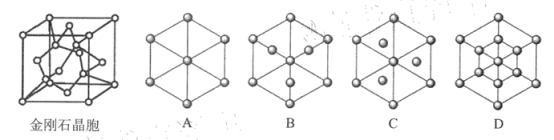
(5)���ʯ��̼��һ��ͬ�������壬����_______ ���塣��֪�����Ƶľ��������������ѻ���������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼ��ͼA ��ʾ������ʯ����������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼӦ������ͼ ___________���� A~D ͼ��ѡ���
��̼ԭ�Ӱ뾶Ϊr ,���ʯ������̼ԭ�ӵĿռ�ռ����Ϊ_____________�� �ú��� �Ĵ���.ʽ��ʾ����
���𰸡�3 4 �縺�Խ�С ԭ�Ӱ뾶�ϴ� ����ˮ�Ķ�����̼����ֻ�в�����ˮ��ϳ�̼�ᣨ�������ɣ� sp3 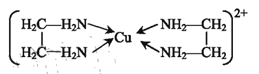 ԭ�� D
ԭ�� D ![]()
��������
(1)ͬһ�ܼ��еĵ���������ͬ����ͬ�ܼ��е���������ͬ��
(2)����������γ����������ʷ������
(3)������ķ��ӽṹ��������̼��ˮ�е��ܽ�ȷ������
(4) ���ݼ۲���ӶԻ���ģ�ͷ�����𣻸�����λ�����γ�ԭ��������λ���ӵĽṹ��
(5)�������ʵ��۷е㡢Ӳ�ȵ����������жϾ������ͣ����ݾ����Ŀռ�ṹ�������
(1)̼ԭ�Ӻ�����6�����ӣ���������Ų�ʽΪ1s22s22p2����1s��2s��2p 3���ܼ����ʺ�����3��������ͬ�ĵ��ӣ����������˶�״̬��4�����ؾ������ֱ�Ϊ��a.���Ӳ㣨��������n����b.�����Dz�͵����Ƶ���״�������������������l����c.�����Ƶ���չ����������m����d.���ӵ�����������������ms����
�ʴ�Ϊ��3��4��
(2)�γ�����������У�1.������縺�Ժܴ��ԭ��A�γ�ǿ���Լ�����ԭ�ӣ�2.���ڽ�С�뾶���ϴ�縺�ԡ����¶Ե��ӡ����в��ָ���ɵ�ԭ��B (F��O��N)��CH4���Ӽ䲻���γ������ ��Ҫԭ����CH4 �����е�̼ԭ�Ӳ����¶Ե��ӡ��縺�Խ�С��ԭ�Ӱ뾶�ϴ�
�ʴ�Ϊ���縺�Խ�С��ԭ�Ӱ뾶�ϴ�
(3)������̼ˮ��Һ������ȴ������ԭ��������ˮ�Ķ�����̼����ֻ�в�����ˮ��ϳ�̼�
�ʴ�Ϊ������ˮ�Ķ�����̼����ֻ�в�����ˮ��ϳ�̼�ᣨ�������ɣ���
(4)�Ҷ�����Nԭ�ӵ��ӻ����������NH3��ͬ�����ݼ۲���ӶԻ���ģ�ͷ���������ԭ��N�ļ۲���Ӷ���Ϊ![]() �������ӻ�����Ϊsp3���Ҷ���ͨ����λ������Cu2+ �γ��ȶ��Ļ�״�����ӣ�Cu2+����λ��Ϊ4������ṹ�ɱ�ʾΪ
�������ӻ�����Ϊsp3���Ҷ���ͨ����λ������Cu2+ �γ��ȶ��Ļ�״�����ӣ�Cu2+����λ��Ϊ4������ṹ�ɱ�ʾΪ ��
��
�ʴ�Ϊ��sp3�� ��
��
(5)���ʯ�۵�ߡ�Ӳ�ȴ�����ԭ�Ӿ��壻���ʯ�ṹ���Ƽ���ռ乹�͵���������ṹ��������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼӦΪ������������Σ�����侧��ͼ��֪Ӧ��ͼD�����ʯ������ͼ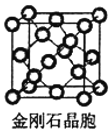 ���þ�����Cԭ�Ӹ���4+8��
���þ�����Cԭ�Ӹ���4+8��![]() +6��
+6��![]() =8�����ʯ��Խ����ϵ��ĸ�ԭ�ӽ��������������ⳤa=
=8�����ʯ��Խ����ϵ��ĸ�ԭ�ӽ��������������ⳤa=![]() ���������=a3=
���������=a3=![]() ������ԭ�����=
������ԭ�����=![]() ���ռ�ռ����=
���ռ�ռ����=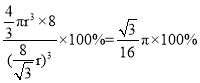 ��
��
�ʴ�Ϊ��ԭ�ӣ�D��![]() ��
��


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����������ò�Ѫ���Ƿ���ʡ�ʵ�鲽�����£�
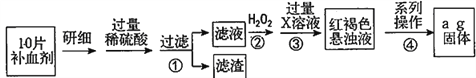
��ش��������⣺
��1��������ٵ���Һ�еμ�KSCN��Һ���Ϊ��ɫ�������Һ�к���______�������ӷ��ţ���
��2���������з�Ӧ�����ӷ���ʽ��__________________________________��
��3���������з�Ӧ�����ӷ���ʽ��__________________________________��
��4����������һϵ�д����IJ������裺���ˡ�______�����ա�_______��������
��5������ʵ���е���ĺ��Բ��ƣ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ______g�����ú�a�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A.�ھƾ��Ƽ��������£�Na2CO3��NaHCO3���嶼�ܷ����ֽ�
B.Cl2��һ���ж����壬������������ˮ��ɱ������
C.SiO2���ܺ�NaOH��Һ��Ӧ���ܺ�����ᷴӦ������������������
D.Na2SiO3ˮ��Һ�׳�ˮ���������Ʊ��轺��ľ�ķ�����ȵ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С���ͬѧ��ѧϰ�˵绯ѧ���֪ʶ������ͼװ�ý���ʵ�飬��ش��������⣺

(1)ʵ��һ��������K��a���ӣ�����Ϊ_____�����缫��ӦʽΪ______��
(2)ʵ��һ�������о�С���ͬѧ�������ҵ缫�����϶����н����е�һ���Է�ֹ������ʴ����ȷ��ѡ����____(����ĸ���)��
A��Cu�� ��B��Zn���� C��Sn�� �� D��Ag
(3)ʵ���������K��b���ӣ�����__�����ܷ�Ӧ�����ӷ���ʽΪ______��
(4)����ʵ���������˵����ȷ����____(����ĸ���)��
A����Һ��Na������ƶ�
B���Ӽ����ݳ���������ʹʪ��ĵ��ۣ�KI��ֽ����
C����Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D����Ӧ�ڽ����缫���ҵ缫���ռ������������һ�����
(5)���о�С���ͬѧ�ڽ���ʵ�����������Һ�еμӷ�̪��Һ������________(����������������)��������졣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£��� 2mol NO2 װ�� 5 L ���������ܱ������У�����Ӧ 2NO2(g) N2O4(g)��H����60 kJ/mol �ﵽƽ��ʱ���ָ���ԭ�����¶ȣ���ô˹�������ϵ����繲�ͷ��� 40 kJ �������� ����˵����ȷ���ǣ� ��
A.�¶����ߣ�ƽ�ⳣ�� K ����
B.����ѹ�����������ƽ�������ƶ������������ɫ��dz
C.������Ӧ�ﵽƽ��ʱ��NO2��ת������ 40%
D.����ʼʱ�������г��� 1.0 mol N2O4�������¶Ȳ��䣬�ﵽƽ��ʱ������ 20 kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£������������Ϊ 1.0 L �ĺ����ܱ������з�����Ӧ��2CH3OH(g)![]() CH3OCH3(g)��H2O(g)
CH3OCH3(g)��H2O(g)
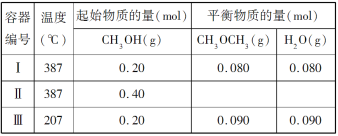
����˵����ȷ���ǣ� ��
A.�÷�Ӧ������ӦΪ���ȷ�Ӧ
B.�ﵽƽ��ʱ���������е� CH3OH ����������������е�С
C.�������з�Ӧ����ƽ������ʱ����������еij�
D.����ʼʱ���������г��� CH3OH 0.15 mol��CH3OCH3 0.15 mol �� H2O 0.10 mol����Ӧ�� ������Ӧ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)��H<0��ij�¶��£��� 2 mol SO2 �� 1 mol O2 ���� 10L �ܱ������У���Ӧ��ƽ���SO2 ��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ���ǣ� ��
2SO3(g)��H<0��ij�¶��£��� 2 mol SO2 �� 1 mol O2 ���� 10L �ܱ������У���Ӧ��ƽ���SO2 ��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ���ǣ� ��
�� ��
�� ��
��
A.��ͼ���ƶϣ�B �� SO3��ƽ��Ũ��Ϊ 0.3molL1
B.��ͼ���У��ڴ��¶��£�C �� �� ������ ��
C.�ﵽƽ�����������䣬���뺤����ѹǿ������Ӧ���ʱ仯ͼ�������ͼ�ұ�ʾ
D.ѹǿΪ 0.50 MPa ʱ����ͬ�¶��� SO2 ��ƽ��ת������ʱ���ϵ��ͼ������ T2>T1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������ء�
I.K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��Cr2O72-(��ɫ)+CH3CH2OH��Cr3+(��ɫ)+CH3COOH(δ��ƽ��
��1����̬Crԭ�ӵļ۵��ӹ������ʽΪ__��
��2��CH3COOH����������Ԫ�صĵ縺���ɴ�С��˳��Ϊ__��̼ԭ�ӵĹ���ӻ�����Ϊ__��������������������Ŀ֮��Ϊ__��
��3����֪Cr3+�ȹ���Ԫ��ˮ�����ӵ���ɫ�����ʾ��
���� | Sc3+ | Cr3+ | Fe2+ | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3+��Zn2+��ˮ������Ϊ��ɫ��ԭ��Ϊ__��
II.ZnCl2Ũ��Һ�����ڳ�ȥ��������������������FeO��Ӧ�ɵ�Fe[Zn(OH)Cl2]2��Һ��
��4��Fe[Zn(OH)Cl2]2��Һ�в����ڵ�������������__(��ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.������ D.��λ�� E.���»��� F.���
��Һ��[Zn(OH)Cl2]-�ĽṹʽΪ__��
III.п������������Ԫ��֮һ����ѻ���ʽ��ͼ1�������ṹ��ͼ2��
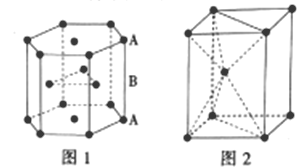
��5��п�Ķѻ���ʽΪ__����λ��Ϊ__��
��6����пԭ�ӵİ뾶Ϊapm�������ӵ�������ֵΪNA����п������ܶ�Ϊ___g/cm3(�ú�a�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���G�Ǻϳ���ũҩ����Ҫ�м��塣�Ի�����AΪԭ�Ϻϳɻ�����G�Ĺ����������£�

��1��������G�к��������ŵ�����Ϊ________��
��2����ӦD��E�ķ�Ӧ����Ϊ________��
��3��������B�ķ���ʽΪC7H6Cl2��B�Ľṹ��ʽΪ______��
��4��д��ͬʱ��������������G��һ��ͬ���칹��Ľṹ��ʽ��______��
���ܷ���������Ӧ��
�ں˴Ź���������ʾ��ԭ�ӵķ�ֵ��Ϊ3��2��2��1��
��5�����Ի�����F��CH2(COOC2H5)2Ϊԭ���Ʊ� ��д���Ʊ��ĺϳ�·������ͼ�����Լ����ã��ϳ�·������ͼʾ����������ɣ���
��д���Ʊ��ĺϳ�·������ͼ�����Լ����ã��ϳ�·������ͼʾ����������ɣ���
__________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com