
| 0.002mol |
| 0.2L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl
���л������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��������HCl ��NaOH ��CH3COOH ��NH3��H2O ��CH3COONa ��NH4Cl
��1������������ʵ��� ����Һ�ʼ��Ե��� ������ţ���
��2��������0.01 mol/L HCl��Һ��PH= ��PH=11��CH3COONa��Һ����ˮ���������c(OH��) = ��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ�� ������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ ��
��4������PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m n ������ڡ����ڡ�С�ڡ�����
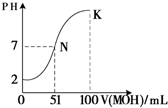
��5�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺ 
����ͼ����Ϣ��֪HAΪ_______�ᣨ�ǿ������������
�� K���Ӧ����Һ�У�
c(M��)��c(MOH)= mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��������HCl ��NaOH ��CH3COOH ��NH3��H2O ��CH3COONa ��NH4Cl
��1������������ʵ��� ����Һ�ʼ��Ե��� ������ţ���
��2��������0.01 mol/L HCl��Һ��PH= ��PH=11��CH3COONa��Һ����ˮ���������c(OH��) = ��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ�� ������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ ��
��4������PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m n ������ڡ����ڡ�С�ڡ�����
��5�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺

����ͼ����Ϣ��֪HAΪ_______�ᣨ�ǿ������������
�� K���Ӧ����Һ�У�
c(M��)��c(MOH)= mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com