
| t/min | X/mol | Y/mol | Z/mol |
| 0 | 1.00 | 1.00 | 0.00 |
| 3 | 0.75 | 0.50 | 0.50 |
| 5 | 0.65 | 0.30 | 0.70 |
| 9 | 0.60 | 0.20 | 0.80 |
| 14 | 0.60 | 0.20 | 0.80 |

| A������ | B������ | C����ѹ | D����ȥ���ַ�Ӧ�� E.���븺���� |
| ʵ�� ��� | �¶�/K | ����Ũ�� /mol��L-1 | ����Ũ��/mol��L-1 | ʵ��Ŀ�� |
| �� | 298 | 0.20 | | a.ʵ��ٺ͢ڣ�̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죻 b.ʵ��ٺۣ͢�̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죻 c.ʵ��ܢݣ�̽���¶ȶ�þ�����ᷴӦ��þ����ᷴӦ���ʵ�Ӱ�죬�ĸ�����һЩ�� |
| �� | 308 | 0.20 | | |
| �� | 298 | 0.40 | | |
| �� | | 0.20 | 0.20 | |
| �� | | | |
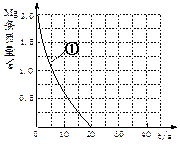
 2Z(g) ��H =+300kJ/mol ��2�֣�
2Z(g) ��H =+300kJ/mol ��2�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H2��g��+
H2��g��+ I2��g���T�THI��g�� ����="+26" kJ��mol-1�������У���ȷ����
I2��g���T�THI��g�� ����="+26" kJ��mol-1�������У���ȷ����| A��1 mol������1 mol��������ȫ��Ӧ��Ҫ����26 kJ������ |
| B��1������Ӻ�1���������ȫ��Ӧ��Ҫ����52 kJ������ |
| C��1 mol H2��g����1 mol I2��g����ȫ��Ӧ����2 mol��HI����������52 kJ������ |
D�� mol H2��g���� mol H2��g���� mol I2��g����ȫ��Ӧ�ų�26 kJ������ mol I2��g����ȫ��Ӧ�ų�26 kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C(s)��O2(g)��CO(g)��H����393.5 kJ/mol |
| B��2H2(g)��O2(g)��2H2O(g)��H����571.6 kJ/mol |
| C��C2H4��g)��3O2(g)��2CO2(g)��2H2O(g)��H����1411.0 kJ/mol |
| D��1/2C6H12O6��s����3O2(g)��3CO2(g)��3H2O(l)��H����1 400 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���ڢۢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C+H2O |
| B��C(s)+H2O(g) |
| C��C(s)+H2O(g) |
D��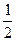 C(s)+ C(s)+ 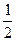 H2O(g) H2O(g)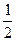 CO(g)+ CO(g)+ 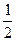 H2(g); ��H=+65.64kJ/mol H2(g); ��H=+65.64kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
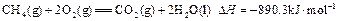 ������0.75mol CH4��CO�Ļ�����壬��ȫȼ�պ�����CO2(g)��18g H2O(l)�����ų�515.9kJ���������Ͽ�֪1mol CO��ȫȼ�շų�������Ϊ�� ��
������0.75mol CH4��CO�Ļ�����壬��ȫȼ�պ�����CO2(g)��18g H2O(l)�����ų�515.9kJ���������Ͽ�֪1mol CO��ȫȼ�շų�������Ϊ�� ��A��283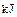 | B��374.4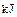 | C��512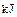 | D��566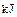 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��CH4 ( g )+2NO2 ( g )= N2( g )+C O2 ( g )+2H2O ( l)��H��һ867 kJ��mol��1 O2 ( g )+2H2O ( l)��H��һ867 kJ��mol��1 |
| B��CH4 ( g )+2NO2 ( g )= N2( g )+CO2 ( g )+2H2O ( g)��H��һ867 kJ��mol��1 |
C��CH4 ( g )+2NO2 ( g )= N2( g )+CO2 ( g )+2H2O ( g)��H��һ586kJ��mol ��1 ��1 |
| D��CH4 ( g )+2NO2 ( g )= N2( g )+CO2 ( g )+2H2O ( g)��H��+586kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1:3 | B��3:1 | C��1:4 | D��1:1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com