
 �����ͼװ��̽��HCl��Һ�������������ڵ糡�е����Ǩ�����ʣ���֪��Cd�Ľ�����Դ���Cu���������£��ڴ�ֱ�IJ���ϸ���ڣ��ȷ�CdCl2��Һ����ɫ����Ȼ��С�ķ���HCl��Һ����aa�䴦�γ������Ľ��森ͨ��ɹ۲쵽�������滺�������ƶ�������˵������ȷ���ǣ�������
�����ͼװ��̽��HCl��Һ�������������ڵ糡�е����Ǩ�����ʣ���֪��Cd�Ľ�����Դ���Cu���������£��ڴ�ֱ�IJ���ϸ���ڣ��ȷ�CdCl2��Һ����ɫ����Ȼ��С�ķ���HCl��Һ����aa�䴦�γ������Ľ��森ͨ��ɹ۲쵽�������滺�������ƶ�������˵������ȷ���ǣ�������| A��ͨ��ɹ۲쵽�������滺�������ƶ���ԭ����Cd2+��Pt�缫Ǩ�ƵĽ�� |
| B��װ����Pt�缫������pH���� |
| C��һ��ʱ���ڣ����ͨ��HCl��Һijһ������ܵ���Ϊ5.0C�����H+��Ǩ�Ƶĵ���Ϊ4.1C��˵����HCl��Һ��H+��Ǩ������Լ��Cl-��4.6�� |
| D�������Դ���������ӣ����¶˲�������Cl2��ʹ���治��������ʵ��ʧ�� |


 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����̿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A������ȫȼ�գ�1mol��ͪ��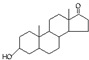 ���ȴ�ͪ�� ���ȴ�ͪ�� ��������3mol O2 ��������3mol O2 |
| B�������顢������������黥Ϊͬ���칹�壬�е��������� |
| C�����ǡ���ѿ�Ǻ����ǵķ���ʽ��ΪC12H22O11�����ܷ���������Ӧ |
| D���Ҵ�����ͨ����ȥ��ȡ�����ӳɷ�Ӧ�������Ҷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����뱽������ɫ����Һ��K+��NH4+��Cl-��I-���ܴ������棨�����£� | ||
B��
| ||
| C����֪��25��ʱ��Mg��OH��2��kSP=5.61��10-12��MgF2��kSP=7.42��10-11��25��ʱ����Mg��OH��2������Һ�м���NaF��Һ��Mg��OH��2����ת��ΪMgF2 | ||
| D����6XeF4+12H2O�T2XeO3+4Xe��+24HF+3O2����Ӧ�У�XeF4����֪��ʽˮ�⣬ÿ����4mol Xe��ת��16mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����0.2mol/Lijǿ��������NaA��Һ��0.1mol/L����������ϣ���Ϻ���Һ�Լ��ԣ�������Һ�� c��HA����c��Cl-����c��A-����c��OH-�� |
| B����ͬ�����£���ˮ������������Һ�зֱ�����������Ȼ�茶��������Һ��pH��ǰ��С���������� |
| C�����������£���pH=3����VaL��pH=11����������ҺVbL��ϣ���Ϻ���ҺpH=4�������Ϻ��������仯����Va��Vb=10��9 |
| D��0.1mol/L pHΪ4��NaHB��Һ�У�c��HB-����c��H2B����c��B2-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��25��ʱ��0.1mol/L HF��Һ��pH=1 |
| B��0.2mol/L MgCl2��Һ������Ũ�ȹ�ϵΪ2c��Mg2+��=c��Cl-����c��H+��=c��OH-�� |
| C��2HF��aq��+Mg2+��aq��?MgF2��s��+2H+��aq�����÷�Ӧ��ƽ�ⳣ��K=1.2��107 |
| D���÷�Ӧ��ϵ����MgF2�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼijѧ�������һ�����ڱ�д���֡���Ȥζʵ�飮��ֽ���������ơ���ɫ��̪�Ļ��Һ��ʪ��Ȼ��ƽ����һ�鲬Ƭ�ϣ���ͨ��Դ����Ǧ��֪��ֽ��д���϶��¡�������ֺ�ɫ�ּ����ݴ�����������ȷ���ǣ�������
��ͼijѧ�������һ�����ڱ�д���֡���Ȥζʵ�飮��ֽ���������ơ���ɫ��̪�Ļ��Һ��ʪ��Ȼ��ƽ����һ�鲬Ƭ�ϣ���ͨ��Դ����Ǧ��֪��ֽ��д���϶��¡�������ֺ�ɫ�ּ����ݴ�����������ȷ���ǣ�������| A��Ǧ�ʶ�����������Ƭ�˷�����ԭ��Ӧ |
| B��Ǧ�ʶ������������� |
| C����������Һ�������������Һ���� |
| D����Ƭ�˷����ĵ缫��ӦΪ��40H--4e-=2H2O+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ�Ⱦ�Ϊ0.1mol?L1�Ĵ�����Һ������������Һ��������C��Na+��=c��CH3COO����c��OH-��=c��H+�� |
| B��Ũ�Ⱦ�Ϊ0.1mol?L-1�����������Һ������������Һ��������C��SO42-����C��Na+����C��NH4+����C��H+����C��OH-�� |
| C��Ũ�Ⱦ�Ϊ0.1mol?L1��С�մ���Һ���ռ���Һ��������C��Na+��+C��H+��=2C��CO32-��+C��OH-�� |
| D��pH=12�İ�ˮ��Һ��pH=2��������Һ��������C��NH4+����C��Cl-����C��OH-����C��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com