
ЁОЬтФПЁПФГ100mLЛьКЯвКжаЃЌHNO3КЭH2SO4ЕФЮяжЪЕФСПХЈЖШЗжБ№ЮЊ0.1mol/LКЭ0.4mol/LЁЃЯђИУЛьКЯвКжаМгШы1.92gЭЗлЃЌМгШШЪЙЗДгІЗЂЩњЭъШЋЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ(КіТдЗДгІЧАКѓШмвКЬхЛ§БфЛЏ)ЃЈ ЃЉ
A.ЫљЕУШмвКжаc(Cu2+)=0.225mol/L
B.ЫљЕУШмвКжаc(H+)=0.5mol/L
C.ЫљЕУЦјЬхдкБъзМзДПіЯТЕФЬхЛ§ЮЊ0.448L
D.ЗДгІжазЊвЦ0.06molЕФЕчзг
ЁОД№АИЁПB
ЁОНтЮіЁП
n(Cu)=![]() =0.03molЃЛШмвКжаn(H+)=0.1mol/LЁС0.1L+0.4mol/LЁС0.1LЁС2=0.09molЃЌn(NO3)=0.1mol/LЁС0.1L=0.01molЃЌЯђЛьКЯШмвКМгШыЭЗлКѓЗЂЩњЗДгІЃК3Cu+8H++2NO3-=3Cu2++2NOЁќ+4H2OЃЌИљОнРызгЗДгІЗНГЬЪНПЩжЊNO3ЕФСПВЛзуЃЌдђЗДгІЕФЭЕФЮяжЪЕФСПЮЊ0.015molЃЌЯћКФЕФЧтРызгЕФЮяжЪЕФСПЮЊ0.04molЁЃ
=0.03molЃЛШмвКжаn(H+)=0.1mol/LЁС0.1L+0.4mol/LЁС0.1LЁС2=0.09molЃЌn(NO3)=0.1mol/LЁС0.1L=0.01molЃЌЯђЛьКЯШмвКМгШыЭЗлКѓЗЂЩњЗДгІЃК3Cu+8H++2NO3-=3Cu2++2NOЁќ+4H2OЃЌИљОнРызгЗДгІЗНГЬЪНПЩжЊNO3ЕФСПВЛзуЃЌдђЗДгІЕФЭЕФЮяжЪЕФСПЮЊ0.015molЃЌЯћКФЕФЧтРызгЕФЮяжЪЕФСПЮЊ0.04molЁЃ
A. ЗДгІЕФЭЕФЮяжЪЕФСПЮЊ0.15molЃЌЫљвдc(Cu2+)=![]() =0.15mol/LЃЌЙЪAДэЮѓЃЛ
=0.15mol/LЃЌЙЪAДэЮѓЃЛ
B. ЗДгІЯћКФЕФЧтРызгЕФЮяжЪЕФСПЮЊ0.04molЃЌдђЪЃгрЕФn(H+)=0.09mol-0.04mol=0.05molЃЌШмвКЬхЛ§ЮЊ100mLЃЌЫљвдХЈЖШЮЊ0.5mol/LЃЌЙЪBе§ШЗЃЛ
C. ИљОнРызгЗНГЬЪНПЩжЊЩњГЩЕФЦјЬхn(NO)= n(NO3)=0.01molЃЌБъПіЯТЬхЛ§ЮЊ0.224LЃЌЙЪCДэЮѓЃЛ
D. ЗДгІЙ§ГЬжа0.01mol NO3БЛЛЙдГЩNOЃЌзЊвЦЕчзгЕФЮяжЪЕФСПЮЊ0.03molЃЌЙЪDДэЮѓЃЛ
ЙЪД№АИЮЊBЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЗДгІЕФРызгЗНГЬЪНЪщаДе§ШЗЕФЪЧ
A. ЯђЫФєЧЛљКЯТСЫсФЦШмвКжаЕЮМгЙ§СПЕФЬМЫсЧтФЦШмвКЃК[Al(OH)4]ЁЊ+4HЃЋ=Al3++2H2O
B. НЋЩйСПSO2ЦјЬхЭЈШызуСПЕФNaClOШмвКжаЃКSO2ЃЋ2ClOЃЃЋH2O=SO32-ЃЋ2HClO
C. NaHSO4ШмвКгыBa(OH)2ШмвКЗДгІжСжаадЃК2HЃЋ+SO42-+Ba2++2OHЁЊ=BaSO4Ё§+2H2O
D. ЯђЗаЫЎжаЕЮМгБЅКЭТШЛЏЬњШмвКЃКFe3++3H2O=Fe(OH)3Ё§+3HЃЋ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ1ЃЉ25 ЁцЪБЃЌжЦБИбЧЯѕѕЃТШЫљЩцМАЕФШШЛЏбЇЗНГЬЪНКЭЦНКтГЃЪ§ШчБэЃК
ШШЛЏбЇЗНГЬЪН | ЦНКтГЃЪ§ | |
Ђй | 2NO2(g)+NaCl(s) | K1 |
Ђк | 4NO2(g)+2NaCl(s) | K2 |
Ђл | 2NO(g)+Cl2(g) | K3 |
дђИУЮТЖШЯТЃЌІЄH3=_______________kJmol-1ЃЛK3=_____________ЃЈгУK1КЭK2БэЪОЃЉЁЃ
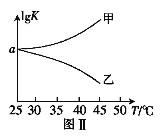
ЃЈ2ЃЉ25ЁцЪБЃЌдкЬхЛ§ЮЊ2LЕФКуШнУмБеШнЦїжаЭЈШы0.08 mol NOКЭ0.04 molCl2ЗЂЩњЩЯЪіЗДгІЂлЃЌШєЗДгІПЊЪМгыНсЪјЪБЮТЖШЯрЭЌЃЌЪ§зжбЙЧПвЧЯдЪОЗДгІЙ§ГЬжабЙЧП(p)ЫцЪБМф(t)ЕФБфЛЏШчЭМЂёЪЕЯпЫљЪОЃЌдђІЄH3 ___ЃЈЬюЁА>ЁБЁА<ЁБЛђЁА=ЁБЃЉ0ЃЛШєЦфЫћЬѕМўЯрЭЌЃЌНіИФБфФГвЛЬѕМўЃЌВтЕУЦфбЙЧПЫцЪБМфЕФБфЛЏШчЭМЂёащЯпЫљЪОЃЌдђИФБфЕФЬѕМўЪЧ_____________ЃЛдк5 minЪБЃЌдйГфШы0.08 mol NOКЭ0.04 molCl2ЃЌдђЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПНЋ_____________ЃЈЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБЃЉЁЃЭМЂђЪЧМзЁЂввСНЭЌбЇУшЛцЩЯЪіЗДгІЂлЕФЦНКтГЃЪ§ЕФЖдЪ§жЕЃЈlgKЃЉгыЮТЖШЕФБфЛЏЙиЯЕЭМЃЌЦфжае§ШЗЕФЧњЯпЪЧ______ЃЈЬюЁАМзЁБЛђЁАввЁБЃЉЃЌaжЕЮЊ__________ЁЃ25 ЁцЪБВтЕУЗДгІЂлдкФГЪБПЬЃЌNO(g)ЁЂCl2(g)ЁЂNOCl(g)ЕФХЈЖШЗжБ№ЮЊ0.8ЁЂ0.1ЁЂ0.3ЃЌдђДЫЪБvе§_________vФцЃЈЬюЁА>ЁБЁАЃМЁБЛђЁА=ЁБЃЉ

(3)дк300 ЁцЁЂ8 MPaЯТЃЌНЋCO2КЭH2АДЮяжЪЕФСПжЎБШ1ЁУ3 ЭЈШывЛУмБеШнЦїжаЗЂЩњCO2(g)ЃЋ3H2(g)![]() CH3OH(g)ЃЋH2O(g)жаЗДгІЃЌДяЕНЦНКтЪБЃЌВтЕУCO2ЕФЦНКтзЊЛЏТЪЮЊ50%ЃЌдђИУЗДгІЬѕМўЯТЕФЦНКтГЃЪ§ЮЊKpЃН_____(гУЦНКтЗжбЙДњЬцЦНКтХЈЖШМЦЫуЃЌЗжбЙЃНзмбЙЁСЮяжЪЕФСПЗжЪ§)ЁЃ
CH3OH(g)ЃЋH2O(g)жаЗДгІЃЌДяЕНЦНКтЪБЃЌВтЕУCO2ЕФЦНКтзЊЛЏТЪЮЊ50%ЃЌдђИУЗДгІЬѕМўЯТЕФЦНКтГЃЪ§ЮЊKpЃН_____(гУЦНКтЗжбЙДњЬцЦНКтХЈЖШМЦЫуЃЌЗжбЙЃНзмбЙЁСЮяжЪЕФСПЗжЪ§)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЗДгІN2(g)+3H2(g)![]() 2NH3(g)ЃЌФГЮТЖШЯТЃЌЯђЬхЛ§ЙЬЖЈЕФ1LУмБеШнЦїжаГфШы1mol N2(g)КЭ3mol H2(g)ЃЌВтЕУВЛЭЌЪБПЬЗДгІЧАКѓЕФбЙЧПЙиЯЕШчЯТБэЫљЪОЃК
2NH3(g)ЃЌФГЮТЖШЯТЃЌЯђЬхЛ§ЙЬЖЈЕФ1LУмБеШнЦїжаГфШы1mol N2(g)КЭ3mol H2(g)ЃЌВтЕУВЛЭЌЪБПЬЗДгІЧАКѓЕФбЙЧПЙиЯЕШчЯТБэЫљЪОЃК
ЪБМф/min | 5 | 10 | 15 | 20 | 25 | 30 |
бЙЧПБШжЕPКѓ/PЧА | 0.98 | 0.88 | 0.80 | 0.75 | 0.75 | 0.75 |
(1)0~15minФк,гУH2БэЪОЕФЦНОљЗДгІЫйТЪЮЊv(H2) =___________________molЁЄL-1ЁЄmin-1ЁЃ
(2)ДяЕНЦНКтЪБN2ЕФзЊЛЏТЪЮЊ________ЃЌИУЮТЖШЯТЕФЦНКтГЃЪ§ЮЊ___________(БЃСєСНЮЛаЁЪ§)ЁЃ
(3)вбжЊИУЗДгІЮЊЗХШШЗДгІЃЌЯТЭМЮЊВЛЭЌЬѕМўЯТЗДгІЫйТЪЫцЪБМфЕФБфЛЏЧщПі(УПДЮНіИФБфвЛИіЬѕМў)ЃКaЪБИФБфЕФЬѕМўПЩФмЪЧ____________ЃЛbЪБИФБфЕФЬѕМўПЩФмЪЧ_______________ЁЃ

(4)вЛЖЈЬѕМўЯТЕФУмБеШнЦїжаЃЌИУЗДгІДяЕНЦНКтКѓвЊЬсИпH2ЕФзЊЛЏТЪЃЌПЩвдВЩШЁЕФДыЪЉгаЃЈ_________ЃЉ
AЃЎЕЭЮТЕЭбЙ BЃЎМгШыДпЛЏМС CЃЎдіМгN2ЕФХЈЖШ DЃЎдіМгH2ЕФХЈЖШ EЃЎЗжРыГіNH3
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПяиЃЈ31GaЃЉЪЧвЛжжживЊН№ЪєдЊЫиЃЌяиМАЦфЛЏКЯЮядкЕчзгЙЄвЕЁЂЙтЕчзгЙЄвЕЁЂЙњЗРЙЄвЕКЭГЌЕМВФСЯЕШСьгђгазХЙуЗКЕФгІгУЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛљЬЌGaдзгеМОнзюИпФмМЖЕчзгЕФЕчзгдЦТжРЊЭМаЮзДЮЊ__________ЃЌЮДГЩЖдЕчзгЪ§ЮЊ________________ЁЃ
ЃЈ2ЃЉGa(NO3)3жавѕРызгЕФСЂЬхЙЙаЭЪЧ_____________ЃЌаДГівЛИігыИУвѕРызгЕФСЂЬхЙЙаЭЯрЭЌЕФЗжзгЕФЛЏбЇЪН___________ЁЃ
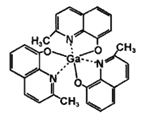
ЃЈ3ЃЉ2-МзЛљ-8-єЧЛљрпјяиЃЈШчЭМЃЉгІгУгкЗжзггЁМЃММЪѕЃЌ2-МзЛљ-8-єЧЛљрпјяижаЮхжждЊЫиЕчИКадгЩДѓЕНаЁЕФЫГађЪЧ____________________________ЃЈЬюдЊЫиЗћКХ)ЃЌЬсЙЉЙТЕчзгЖдЕФГЩМќдзгЪЧ_____________ЁЃ
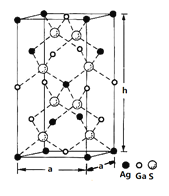
ЃЈ4ЃЉвЛжжЙшяиАыЕМЬхВФСЯЕФОЇАћНсЙЙШчЭМЫљЪОгЩСђЁЂяиЁЂвјаЮГЩЕФЛЏКЯЮяЕФОЇАћЪЧЕзУцЮЊе§ЗНаЮЕФГЄЗНЬхЃЌНсЙЙШчЯТЭМЫљЪОЃЌдђИУОЇЬхжаСђЕФХфЮЛЪ§ЮЊ___________ЃЌОЇАћЕзУцЕФБпГЄa=5.75 nmЃЌИпh=10.30nmЃЌИУОЇЬхУмЖШЮЊ__________________gЁЄcm-3ЃЈСаГіМЦЫуЪНМДПЩЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПУїЗЏЪЏЪЧжЦШЁМиЗЪКЭЧтбѕЛЏТСЕФживЊдСЯЁЃУїЗЏЪЏЕФзщГЩКЭУїЗЏЯрЫЦЃЌДЫЭтЛЙКЌгабѕЛЏТСКЭЩйСПбѕЛЏЬњдгжЪЁЃОпЬхЪЕбщВНжшШчЭМЫљЪОЃК
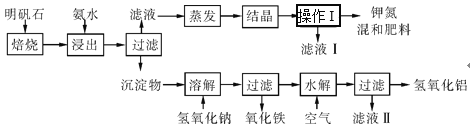
ИљОнЩЯЪіЭМЪОЃЌЭъГЩЯТСаЬюПеЁЃ
ЃЈ1ЃЉУїЗЏЪЏБКЩеКѓгУЯЁАБЫЎНўГіЁЃЪЕбщашвЊ500mLЯЁАБЫЎ(УПЩ§КЌга19.60gАБ)ашвЊШЁХЈАБЫЎ(УПЩ§КЌга250.28gАБ)___mLЃЌгУЙцИёЮЊ___mLСПЭВСПШЁЁЃ
ЃЈ2ЃЉаДГіГСЕэЮяжаЫљгаЮяжЪЕФЛЏбЇЪНЃК___ЁЃ
ЃЈ3ЃЉВйзїЂёЕФУћГЦЪЧ___ЃЌЫљгУЕФВЃСЇвЧЦїга___ЁЃ
ЃЈ4ЃЉЮЊВтЖЈЛьКЯЗЪСЯK2SO4ЁЂ(NH4)2SO4жаМиЕФКЌСП(вдK2OМЦ)ЃЌЭъЩЦЯТСаВНжшЃК
ЂйГЦШЁМиЕЊЗЪЪдбљВЂШмгкЫЎЃЌМгШызуСПBaCl2ШмвКЃЌВњЩњ___ЁЃ
Ђк___ЁЂ___ЁЂ___(вРДЮЬюаДЪЕбщВйзїУћГЦ)ЁЃ
ЂлРфШДЁЂГЦжиЁЃ
ЂмШєЪдбљЮЊmgЃЌГСЕэЕФЮяжЪЕФСПЮЊnmolЃЌдђЪдбљжаМиЕФКЌСП(вдK2OМЦ)ЮЊ___%ЃЈжЪСПЗжЪ§ЃЉ(гУКЌmЁЂnЕФДњЪ§ЪНБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЁЂEЁЂFЮЊдЊЫижмЦкБэжаЧАЫФжмЦкдЊЫиЃЌЧвдзгађЪ§вРДЮдіДѓЃЌAгыЦфгрЮхжждЊЫиМШВЛЭЌжмЦквВВЛЭЌжїзхЃЌBЕФвЛжжКЫЫидкПМЙХЪБГЃгУРДМјЖЈвЛаЉЮФЮяЕФФъДњЃЌCЕФбѕЛЏЮяЪЧЕМжТЫсгъЕФжївЊЮяжЪжЎвЛЃЌDдзгКЫЭтЕчзгга8жжВЛЭЌЕФдЫЖЏзДЬЌЃЌEЕФЛљЬЌдзгдкЧАЫФжмЦкдЊЫиЕФЛљЬЌдзгжаЕЅЕчзгЪ§зюЖрЃЌFдЊЫиЕФЛљЬЌдзгзюЭтФмВужЛгавЛИіЕчзгЃЌЦфЫћФмВуОљвбГфТњЕчзгЁЃ
ЃЈ1ЃЉаДГіЛљЬЌEдзгЕФМлЕчзгХХВМЪН_______ЁЃ
ЃЈ2ЃЉAгыCПЩаЮГЩCA3ЗжзгЃЌИУЗжзгжаCдзгЕФдгЛЏРраЭЮЊ______ЃЌИУЗжзгЕФСЂЬхНсЙЙЮЊ_____ЃЛCЕФЕЅжЪгыBDЛЏКЯЮяЪЧЕШЕчзгЬхЃЌОнЕШЕчзгЬхЕФдРэЃЌаДГіBDЛЏКЯЮяЕФЕчзгЪН______ЃЛA2DгЩвКЬЌаЮГЩОЇЬхЪБУмЖШМѕаЁЃЌЦфжївЊдвђЪЧ__________ЃЈгУЮФзжа№ЪіЃЉЁЃ
ЃЈ3ЃЉвбжЊDЁЂFФмаЮГЩвЛжжЛЏКЯЮяЃЌЦфОЇАћЕФНсЙЙШчЭМЫљЪОЃЌдђИУЛЏКЯЮяЕФЛЏбЇЪНЮЊ___ЃЛШєЯрСкDдзгКЭFдзгМфЕФОрРыЮЊa cmЃЌАЂЗќМгЕТТоГЃЪ§ЕФжЕЮЊ![]() NAЃЌдђИУОЇЬхЕФУмЖШЮЊ______gЁЄcmЃ3ЃЈгУКЌaЁЂNAЕФЪНзгБэЪОЃЉЁЃ
NAЃЌдђИУОЇЬхЕФУмЖШЮЊ______gЁЄcmЃ3ЃЈгУКЌaЁЂNAЕФЪНзгБэЪОЃЉЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкШнЛ§вЛЖЈЕФУмБеШнЦїжаЃЌжУШывЛЖЈСПЕФ NO(g)КЭзуСПC(s)ЃЌЗЂЩњЗДгІ C(s)ЃЋ2NO(g)![]() CO2(g)ЃЋN2(g)ЃЌЦНКтзДЬЌЪБ NO(g)ЕФЮяжЪЕФСПХЈЖШ c(NO)гыЮТЖШ T ЕФЙиЯЕШчЭМЫљЪОЁЃдђЯТСаЫЕЗЈжае§ШЗЕФЪЧ( )
CO2(g)ЃЋN2(g)ЃЌЦНКтзДЬЌЪБ NO(g)ЕФЮяжЪЕФСПХЈЖШ c(NO)гыЮТЖШ T ЕФЙиЯЕШчЭМЫљЪОЁЃдђЯТСаЫЕЗЈжае§ШЗЕФЪЧ( )
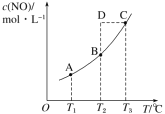
A.ИУЗДгІЕФ ІЄH>0B.ШєИУЗДгІдк T1ЁЂT2 ЪБЕФЦНКтГЃЪ§ЗжБ№ЮЊ K1ЁЂK2ЃЌдђ K1<K2
C.дк T3 ЪБЃЌШєЛьКЯЦјЬхЕФУмЖШВЛдйБфЛЏЃЌдђПЩвдХаЖЯЗДгІДяЕНЦНКтзДЬЌ CD.дк T2 ЪБЃЌШєЗДгІЬхЯЕДІгкзДЬЌDЃЌдђДЫЪБвЛЖЈга v е§<v Фц
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПH2AЪЧЖўдЊШѕЫсЃЌ25ЁцЪБЃЌХфжЦвЛзщc(H2A)ЃЋc(HAЃ)ЃЋc(A2Ѓ)ЃН0.1molЁЄL-1ЕФH2AКЭNaOHЛьКЯШмвКЃЌШмвКжаH2AЁЂHAЃКЭA2ЃЫљеМШ§жжСЃзгзмЪ§ЕФЮяжЪЕФСПЗжЪ§ЃЈІСЃЉЫцШмвКpHБфЛЏЕФЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
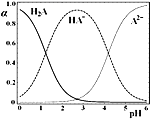
A.c(NaЃЋ)ЃН0.1molЁЄL-1ЕФШмвКжаЃКc(HЃЋ)ЃНc(A2Ѓ)ЃЋc(OHЃ)Ѓc(H2A)
B.c (HAЃ)ЃНc(A2Ѓ)ЕФШмвКжаЃКc(NaЃЋ)ЃО3c(A2Ѓ)
C.c (HAЃ)ЃН0.5molЁЄL-1ЕФШмвКжаЃК2c(H2A)ЃЋc(HЃЋ)ЃНc(OHЃ)ЃЋ1.5molЁЄL-1
D.pHЃН2ЕФШмвКжаЃКc(HAЃ)ЃЋ2c(A2Ѓ)ЃМ0.1
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com