
����Ŀ��
AlN ����������Ҫ�İ뵼����ϣ�Ga(��)��P��As(��)�����γɻ�����뵼����ϵ���ҪԪ�ء��ش��������⣺
(1)As��̬ԭ�ӵĵ���ռ����______���ܲ㣬����ܼ��ĵ����Ų�ʽΪ______����Asλ��ͬһ���ڣ���δ�ɶԵ�����Ҳ��ͬ��Ԫ�ػ���______�֡�
(2)Ԫ�����ڱ��У���P���ڵ�4��Ԫ���е縺��������______ (��Ԫ������)��Si��P��S����Ԫ�ص� ��һ�������ɴ�С��˳����______��
(3)NH3��PH3��AsH3���ߵķе��ɸߵ��͵�˳����______��ԭ����______��
(4)��������P4�����γɵķ��Ӿ��壬P4���ӳ���������ṹ��Pԭ��λ������������ĸ����㣬��Pԭ�ӵ��ӻ���ʽΪ_____������������CS2��������ˮ��ԭ����__________________��
(5)����GaxIn1-xAs(������)�Ȳ��ϣ������̫���ܵ�ص�Ч�ʡ�GaxIn1-xAs�������ξ�����ÿ����������Ķ���һ��ԭ�ӣ������ڲ���4 ��ԭ�ӣ���þ����к���_________����ԭ�ӡ�
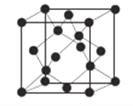
(6)AlN����ľ����ṹ����ʯ����(����ͼ)���辧���ı߳�Ϊ�� pm��NA��ʾ�����ӵ���������þ�����ܶ�Ϊ__________g��cm-3��
���𰸡� 4 4p3 2 N P>S>Si NH3>AsH3>PH3 NH3���Ӵ����������е��AsH3��PH3�ĸߣ�AsH3��PH3���Ӽ䲻���������AsH3����Է����������Ӽ�����������е��PH3�ĸ� sp3 P4��CS2���ǷǼ��Է��ӣ�H2O�Ǽ��Է��ӣ�������������ԭ����P4������CS2��������ˮ 4 ![]()
����������1����������Ų�ʽ����д���ܼ������ߵ͵��жϵȣ�Asλ�ڵ�������VA�壬�����Ų�ʽΪ1s22s22p63s23p63d104s24p3������ռ����4���ܲ㣬����ܼ���4p���������ܼ��ĵ����Ų�ʽΪ4p3��Asδ�ɶԵ�����Ϊ3����˵�������δ�ɶԵ�����Ϊ3��Ԫ����V��Co����2��Ԫ�أ���2������縺�Թ��ɡ���һ�����ܵĹ��ɣ�P�����ڵ�4��Ԫ�طֱ���Si��S��N��As��ͬ���ڴ������ҵ縺������ͬ������ϵ��µ縺�Լ�С��N�ĵ縺�Դ���S��������ڵ�4��Ԫ�ص縺��������N��ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA����˵�һ�����ܵĴ�С˳����P>S>Si����3�������۷е�ߵ͵��жϣ�N��P��As����ͬһ���壬NH3���Ӽ���ڷ��Ӽ���������������������Ӽ���������NH3�ķе�����������ߣ�AsH3����Է�����������PH3��AsH3�ķ��Ӽ�����������PH3��AsH3�ķе����PH3������С˳����NH3>AsH3>PH3����4�������ӻ������жϡ��ܽ��ԣ�P4�ĽṹʽΪ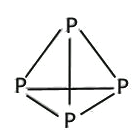 ��P��3��������1���µ��Ӷԣ�����ӻ�����Ϊsp3��CS2Ϊ�Ǽ��Է��ӣ�P4Ϊ�Ǽ��Է��ӣ�H2OΪ���Է��ӣ������������ܣ�����������CS2��������ˮ����5��ÿһ����������Ķ���һ��ԭ�ӣ������ڲ���4��ԭ�ӣ���������ԭ������Ϊ8��1/8��6��1/2��4=8,�ɻ�ѧʽ��֪������As��Ŀռԭ��������1/2����������As��ԭ����ĿΪ8��1/2=4����6�����龧���ļ��㣬����AlN�ľ����Ľṹ���þ���������Ϊ
��P��3��������1���µ��Ӷԣ�����ӻ�����Ϊsp3��CS2Ϊ�Ǽ��Է��ӣ�P4Ϊ�Ǽ��Է��ӣ�H2OΪ���Է��ӣ������������ܣ�����������CS2��������ˮ����5��ÿһ����������Ķ���һ��ԭ�ӣ������ڲ���4��ԭ�ӣ���������ԭ������Ϊ8��1/8��6��1/2��4=8,�ɻ�ѧʽ��֪������As��Ŀռԭ��������1/2����������As��ԭ����ĿΪ8��1/2=4����6�����龧���ļ��㣬����AlN�ľ����Ľṹ���þ���������Ϊ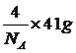 �����������Ϊ(a��10��10)3cm3�������ܶȵĶ��壬�ܶ�Ϊ
�����������Ϊ(a��10��10)3cm3�������ܶȵĶ��壬�ܶ�Ϊ![]() g/cm3��
g/cm3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У�ǰ�߸պ��Ǻ����������� (����)
A. 2 mol H2O��Ħ��������1 mol H2O��Ħ������
B. 200 mL 1 mol��L-1�Ȼ�����Һ��c(Cl-)��100 mL 2 mol��L-1�Ȼ�����Һ��c(Cl-)
C. 64 g������������ԭ�����ͱ�״����22.4 Lһ����̼����ԭ����
D. 20% NaOH��Һ��NaOH�����ʵ���Ũ�Ⱥ�10% NaOH��Һ��NaOH�����ʵ���Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺

��1���á�84����Һ�������ʵ���Ũ��ԼΪ____________mol��L��1��������λ��Ч���֣���
��2��ijͬѧȡ100 mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)��________ mol��L��1��
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ37.25%������Һ������˵����ȷ����________(����ĸ)��

A����ͼ��ʾ�������У��������Dz���Ҫ�ģ������Ҫһ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɺ����������Һ����
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
D����Ҫ����NaClO���������Ϊ214.6 g
��4����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ҫ500mL 1.2mol��L��1��ϡ���ᣬ����ԭ��Ϊ98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ���ᡣ
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________ mol��L��1��
��������Ͳ��ȡŨ��������Ϊ________mL��
������ƿʹ��ǰ�����Ƿ�©ˮ��Ӧ��μ��__________________________��
�����в�����ʹ����ϡ����Ũ��ƫ�ߵ���______________��
A��ȡ��Ũ����ʱ���ӿ̶���
B������ƿ������ˮϴ�Ӻ���1.2mol/L��������ϴ
C��ת����Һʱ��������������Һ����
D������ʱ��������ƿ�̶���
E����ϡ�ͺ��ϡ��������ת������ƿ�ҽ��к����ʵ�����
F�����ݺ�����ƿ����ҡ�ȣ����ź���Һ����ڿ̶��ߣ��ֲ��伸�� ����ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������ϢϢ��ء�����������ȷ����
A. ��ե��֭����ˮ����� B. ��ͷԽ��Խ��
C. ��֬��ˮ��ɱ�������� D. �����еĵ��۾�ˮ��ɱ�ɾ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ӫ����Ƭ���ж��ֳɷ֣���������������
A. ���� B. �� C. Na2HPO4 D. ��ɰ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����������������鼰���ǵĻ�����㷺Ӧ���ڳ�������ϵ�������ش��������⣺
��1��Fe2+�ĺ�������Ų�ʽΪ_________________��
��2��NH3��һ�ֺܺõ����壬NH3�ķе�______(����������������������������)AsH3��
��3����ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ������ͼ�����߱�ʾ��������Ϊ________________��

��4������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡�Ni(CO)4������________(����)��
A��ˮ����B�����Ȼ�̼ C���� D����������Һ
��5��As��±������۵�������
AsCl3 | AsBr3 | AsI3 | |
�۵�/K | 256.8 | 304 | 413 |
����±�����۵�����ԭ����________________��
��6����FeCl3��Һ�е���EDTA�Լ��ɵ������A,��ṹ��ͼ��ʾ��ͼ��M����Fe3+����Fe3+�뵪ԭ��֮���γɵĻ�ѧ����_________��Fe3+����λ��Ϊ_________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ�� 100 �������֮�ȣ���ʹ�á���ش��������⣺

��1���á�84����Һ�������ʵ���Ũ��ԼΪ________ mol��L1������������ 2λ��Ч���֣���
��2��ijͬѧȡ 100mL �á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ�� c(Na��)��_______ mol��L1��
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO�������� 480mL��NaClO��������Ϊ25%������Һ������˵����ȷ����______������ĸ����
A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������
B�� �������õ�NaClO�������ձ����ܽ��Ӧ����ת������ƿ����ˮ���̶���
C�� ����ʱ��������ƿ�̶��ᵼ��������ҺŨ��ƫ��
D����Ҫ���� NaClO���������Ϊ 143.0g

��4����ͬѧ������ƿ����ʹ�÷�������ʶ����ȷ����________
A������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
B������ƿ������ˮϴ���� ��Ҫ��ɺ���ʹ��
C��������Һ�����У�����ƿ��Һ��ֻ��Ҫһ��ҡ�ȹ���
D��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
��5����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա�� 98%���ܶ�Ϊ1.84g��cm3����Ũ�������� 2L 2.3mol��L1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ_______ mol��L1��
������Ũ��������Ϊ_______mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����Ӧ���������ڹ�ҵ�������ǣ� ��
A.��̿�ڸ����»�ԭ���������ƴֹ�
B.��������������ƽ�����
C.������̼���������Ʒ�Ӧ��̼����
D.������ʯ���鷴Ӧ��Ư��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£������ºϳɲ���ش����⣺
��֪��C6H6������+Br2���壩��C6H5Br���屽��+HBr������Ҫ��������������
Һ�塢�廯��������������Ʒ�Ӧ�������屽�����������Ʒ�Ӧ���廯��������ˮ��

�� | �� | �屽 | |
�ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
��1������c������_________��
��2����a�м���15 mL��ˮ����������м����b��С�ļ���4.0 mL Һ̬�塣��a�е��뼸���壬�а�������������Ϊ������__________���壬�����μ���Һ����ꡣװ��d��������____________________��
��3��Һ�����������в�������ᴿ��
����a�м���10 mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10 mLˮ��8 mL10����NaOH��Һ��10 mLˮϴ�ӡ�NaOH��Һϴ�ӵ�������_____________________���ڶ���ˮϴ��Ŀ��Ϊ________________��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ������Ȼ��Ƶ�Ŀ����______��
��4��������������������屽�л����в������ʣ�Ҫ��һ���ᴿ��ѡ���װ��Ϊ___��������ȷѡ��ǰ����ĸ����

��5���ڸ�ʵ���У�a���ݻ����ʺϵ���________��������ȷѡ��ǰ����ĸ����
A��25 mL B��50 mL C��250 mL D��500 mL
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com