
����Ŀ�����ֶ���������Ԫ��X��Y��Z��W��ԭ���������������������X��Y��Z����Ԫ����ɣ�25��ʱ��0.01mol/L ����Һ�е�c(OH-)/c(H+)=1010��Z��W ͬ���ڣ���W������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ4������˵������ȷ����
A. �����ʵ����Ļ�����Z2Y2 ��Z2W�������Ӹ�����ͬ
B. ԭ�Ӱ뾶X
C. մ��W�ĵ��ʵ��Թܿ��þƾ�ϴ��
D. ���⻯����ȶ���Y
���𰸡�A
�����������ֶ���������Ԫ��X��Y��Z��W��ԭ���������������������X��Y��Z����Ԫ����ɣ�25��ʱ��0.01molL-1����Һ��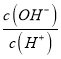 =1010����֪c(OH-)=0.01mol/L�����ΪNaOH�����ԭ��������֪XΪH��YΪO��ZΪNa��Z��Wͬ���ڣ���W���������������۵Ĵ�����Ϊ4�������Ϊ+6�ۣ���֪WΪS��������������֪��XΪH��YΪO��ZΪNa��WΪS��A. �������ƺ������е������ӷֱ�Ϊ���������Ӻ������ӣ������ʵ����Ļ�����������ƺ����Ƶ������Ӹ�����ͬ����A��ȷ��B�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴӴ�������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��X��Y��W��Z����B����C��S���ھƾ���������CS2��ӦѡCS2ϴ�ӣ���C����D���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ������⻯����ȶ��ԣ�Y��W����D��������ѡA��
=1010����֪c(OH-)=0.01mol/L�����ΪNaOH�����ԭ��������֪XΪH��YΪO��ZΪNa��Z��Wͬ���ڣ���W���������������۵Ĵ�����Ϊ4�������Ϊ+6�ۣ���֪WΪS��������������֪��XΪH��YΪO��ZΪNa��WΪS��A. �������ƺ������е������ӷֱ�Ϊ���������Ӻ������ӣ������ʵ����Ļ�����������ƺ����Ƶ������Ӹ�����ͬ����A��ȷ��B�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ���ڴӴ�������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��X��Y��W��Z����B����C��S���ھƾ���������CS2��ӦѡCS2ϴ�ӣ���C����D���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ������⻯����ȶ��ԣ�Y��W����D��������ѡA��


 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�Ka (HCOOH)=1.77��10-4��Ka (CH3COOH)=1.75��10-5��Kb (NH3��H2O) =1.76��10-5������˵����ȷ������������
A. ��CH3COONa��Һ��20��������30������Һ��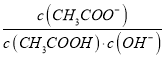 ����
����
B. ����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ������pH��Ϊ3��HCOOH��CH3COOH��Һ���յ㣬����NaOH��Һ��������
C. 0.2 mol/L HCOOH �� 0.1 mol/L NaOH �������Ϻ���c(HCOO-) + c(OH-) = c(HCOOH) + c(H+)
D. 0.2 mol/L CH3COONa �� 0.1 mol/L����������Ϻ� (pH<7)��c(CH3COO-) > c(Cl- ) >c(CH3COOH) >c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��Ҫ����450 mL 0.5 mol��L��1��NaOH��Һ����ش��������⣺
��1����ʵ��������õ��IJ��������У�����������Ͳ���ձ���______________����ͷ�ιܡ��Լ�ƿ��
��2����������ƽ����ʱ��Ӧ��NaOH����________��������ȡ�Ĺ�������Ϊ_______��
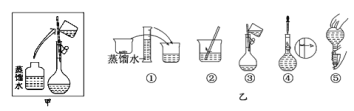
��3������ʱ������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е�___(��ѡ����ĸ)֮�䡣
A������ڡ� B������ۡ� C������ܡ� D�������
��4�����ƹ�����ϴ���ձ���������2��3�ε�Ŀ����______________________��
��5�����ݵμ�����ˮʱ�������������˿̶��ߣ������ķ�����______________��
��6����ͬѧʵ������NaOH��Һ��Ũ��Ϊ0.6 mol��L��1��ԭ�������____(�����)��
a��������������
b��ϴ��������ƿ�в���������ˮ
c������NaOH����ʱ�������ˡ��������
d������ʱ���ӿ̶���
e������ҡ�Ⱥ���Һ����ڿ̶����ּ�������ˮ�����̶���
f���ܽ������ձ���Һ��δϴ��
g������ǰ��Һδ������ȴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017�꣬�п�Ժ������ѧ�����о���̼��ԴС�������������ô��������о��飨DNL19T3���」��������о�Ա�Ŷ�ͨ�����һ������Na-Fe3O4/HZSM-5��ܸ��ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ������ֵ���͡����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
(1) ��ҵ����β���е�CO2������֮һ�ǰ�ˮ��Һ���ռ��������������ǽ�������ȴ��15.5��26.5����ð�ˮ���չ�����CO2���÷�Ӧ�Ļ�ѧ����ʽΪ_____________�����ð�ˮ����ǰ����������ȴ��15.5��26.5��Ŀ���ԭ����_________________��
(2) ���о��ɹ���CO2��ȡ������ֵ���͵�·�����Ƚ�CO2����ˮú����Ӧ����CO��Ȼ����CO��H2��Ӧ���������������������ռ�����������İٷֱȽ�80%��
�� ��֪��H2 (g)��![]() O2 (g) === H2O(l) ��H1= ��285.8 kJ��mol-1
O2 (g) === H2O(l) ��H1= ��285.8 kJ��mol-1
C8H18(l)��![]() O2(g) === 8CO2(g)��9H2O(l) ��H3= ��5518 kJ��mol-1
O2(g) === 8CO2(g)��9H2O(l) ��H3= ��5518 kJ��mol-1
��д��25�桢101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)���Ȼ�ѧ��
��ʽ__________________________��
�� ��һʵ�������г���һ������CO2��H2�����������ǡ����ȫ��Ӧ���Ҳ���ֻ����C5���ϵ��������ʺ�ˮ����CO2��H2�����ʵ���֮�Ȳ�����________��
(3) ������(CH3OCH3)��ʮ����ֵ�ߣ�ȼ��β������Ⱦ���٣��ɴ�����͡�CO��CO2��ϼ���ķ�������һ����Ӧ���н��ϳ���ֱ��ת��Ϊ�����ѣ���������4����Ӧ��
CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��
CH3OH(g)��H2O(g) ��
CO2(g)��H2(g)![]() CO(g)��H2O(g) �� ��
CO(g)��H2O(g) �� ��
CO(g)��2H2(g)![]() CH3OH(g) ��
CH3OH(g) ��
2CH3OH(g)![]() CH3OCH3(g)��H2O(g) ��
CH3OCH3(g)��H2O(g) ��
�� ��֪��Ӧ����ij�¶��µ�ƽ�ⳣ��ΪK=400�����¶��£���һ�����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/��mol��L��1�� | 0.44 | 0.6 | 0.6 |
��ʱ�����淴Ӧ���ʵĴ�С��v(��) ______ v(��) ���>������<������)��
������CH3OH��10 min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v(CH3OH)��_____��
�� ij����С���ڷ�Ӧ�¶�503K��543K����Ӧѹǿ�ֱ���3��4��5��6��7MPa��n(CO)/n(CO+CO2)=0.5������¼����˶����ѵ�ƽ������(������ʵ�ʲ����뷴Ӧ������֮��)�������ͼ1��ʾ��

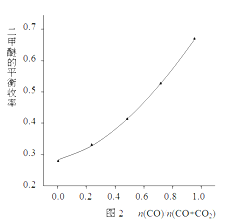
a~e���߷ֱ��Ӧ��ͬ��ѹǿ��������e��Ӧ��ѹǿ��______��������___________��
�ÿ���С���ڷ�Ӧ�¶�523K����Ӧѹǿ5MPa�������£����պϳɷ�Ӧ�Ļ�ѧ�����Ƚ��ϣ��ı�n(CO)/n(CO+CO2)�ı����������ѵ�ƽ�����ʵı仯������ͼ2��ʾ��
��ͼ2��֪�������ѵ�ƽ����������n(CO)/n(O+CO2)��ֵ�������������������ԭ����________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����᳦��ҩ����Ч�ɷ�M�ĺϳ�·������(���ַ�Ӧ��ȥ�Լ�������)

��֪����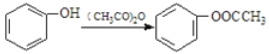 ��
��
��1����A��������_________________��G�еĹ�����������_______________________��
��2����Ӧ�ٵķ�Ӧ������__________________����Ӧ�ڵķ�Ӧ������_____________________��
��3�� E������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��______________________________________��
��4����������������D��ͬ���칹����_______�֡����к˴Ź���������4����ҷ����֮��Ϊ6:2:1:1�Ľṹ��ʽ��_____________________��(д��һ�ּ���)
�����ڷ����廯�����ұ�������3��ȡ����
���ܺ�NaHCO3��Һ��Ӧ��������
��5����֪![]() �ױ��������������������ʱ������һ��ȡ��������ȡ����������ڶ�λ���������ϵĺϳ�·�ߣ����һ����A Ϊԭ�Ϻϳɻ�����
�ױ��������������������ʱ������һ��ȡ��������ȡ����������ڶ�λ���������ϵĺϳ�·�ߣ����һ����A Ϊԭ�Ϻϳɻ�����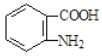 �ĺϳ�·��_______________
�ĺϳ�·��_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ij������������һ�����͵��ɣ�BΪ��գ�A���ѳ���2molSO2��lmolO2����һ�������·������淴Ӧ2SO2+O2![]() 2SO3����H=-QkJ/mol����Q>0)����ʱ�����������־�ֹ��SO2�ķ�Ӧ����ΪY0������A��Ѹ�ٳ���2 molSO2��lmolO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����ΪY���ڴ˹����У�����˵����ȷ����
2SO3����H=-QkJ/mol����Q>0)����ʱ�����������־�ֹ��SO2�ķ�Ӧ����ΪY0������A��Ѹ�ٳ���2 molSO2��lmolO2���������ٴα��־�ֹʱ��SO2�ķ�Ӧ����ΪY���ڴ˹����У�����˵����ȷ����
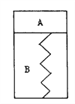
A. ���������ƣ������Ƶ�ԭ��ֹλ�ã�Y>Y0
B. ���������ƣ������Ƶ�ԭ��ֹλ�ã�Y = Y0
C. ���������ƣ������Ƶ�����ԭ��ֹλ�ã�Y>Y0
D. ���������ƣ������Ƶ�����ԭ��ֹλ�ã�Y = Y0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ƽ���з���ʽ
��___FeCl3 ��___KI===____FeCl2��____KCl��_____I2
��____ClO����_____Fe(OH)3��_____OH-===_____Cl����____FeO![]() ��____H2O
��____H2O
��2����Ũ��������ϡ����Ĺ����У����в�����ʹ���Ƶ�ϡ������ҺŨ��ƫ�ߵ���___������ţ���
����ȡŨ�������Ͳ������ˮϴ��2��3�Σ�����ϴ��Һת������ƿ
������ƿʹ��ʱδ����
���ܽ��δ����ȴ����Һ������
�ܶ���ʱ��С������������ˮ�ε�ƿ��
�ݶ��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��зḻ�ĺ�ˮ��Դ�����������ú�ˮ��Դ�ǵ�ǰ��ѧ�о���һ����Ҫ������ͼ�Ǻ�ˮ�ۺ����õ�һ�����档

��.��1������������û���漰�����Ĵ������Ӧ������ ______��
a�����Ϸ�Ӧ b���ֽⷴӦ c���û���Ӧ d�����ֽⷴӦ
��2������1��������������___________��
��3�������к���Ca2����Mg2����SO42�������ʣ�����ʱ�����Լ�Ϊ��
a.���� b.BaCl2��Һ c.NaOH��Һ d.Na2CO3��Һ
�����Լ���˳����_____________��
II.ʵ�������þ�������480mL 2.0mol��L1NaCl��Һ��
��4��������ƿ�⣬����Ҫ�IJ���������___________________��
��5����������ƽ��ȡ����NaCl________g��
��6������ʱ�������¼���������У��ټ��� �ڳ��� ���ܽ� ����ȴ ��ת�� ���� ��ҡ�� ��װƿ�������л�ȱ��һ����Ҫ������______________________��
��7�����д��������ʹ�������Ȼ�����ҺŨ��ƫ�͵�����_________��
a������ƿϴ��������˲��ֵ�ˮ
b��ת��ʱ��Һ��������ƿ����
c������ʱ��������ƿ�Ŀ̶���
d��ҡ�Ⱥ��Һ���½����ټ�ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ԫ�ؼ��䵥�ʴ�Li��Cs�����ʵݱ������ȷ����
A.�ܶ�������B.�۷е�������
C.����������ǿD.��ԭ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com