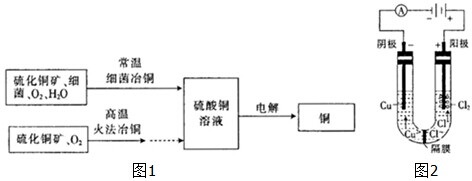
��ͼ1Ϊϸ��ұͭ�ͻ�ұﴵ���Ҫ���̣�
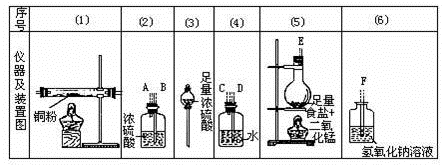
��1������ͭ��Һһ���
����ᡱ��������С����ԣ�ԭ����
�������ӷ���ʽ��ʾ����д���������ͭ��Һ�Ļ�ѧ����ʽ��
���������У�ʼ����������������
��2��ϸ��ұ���ֳ���������ǽ���ʪ��ұ��ҵ�ϵ�һ���¹��գ�ϸ��ұͭ���ұͭ��ȣ��ŵ�Ϊ
��д��һ�㼴�ɣ���
��3���ö��Ե缫�ֱ���Ũ���Ȼ�ͭ��Һ������ͭ��Һ�����Ũ���Ȼ�ͭ��Һʱ���� �����н���ͭ���ɣ�ͬʱ��������������غ�ɫ��Һ�����������ͭ��Һʱ��û���غ�ɫ��Һ���ɣ���ͼ2�ǹ����غ�ɫ��Һ�ɷֵ�̽��
������ͬѧ��Ϊ�������������ֵ��غ�ɫ��Һ��������Ӧ�Ľ��������Ϊ���IJ²��� ����ȷ��
�����ȷ������ȷ������ԭ����
����1��һ����л�ϼ�̬��ָ��������ͬһԪ�ش������ֲ�ͬ�Ļ��ϼۣ���Fe
3O
4�е� FeԪ���������ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫҪ�
����2��CuCl����ˮ��������Ũ���ᣮ
�ڲ��룺�غ�ɫ��Һ�п��ܺ��ӵ�������
����3����Ҫ���ӷ��ţ���
����֤���룺���ʵ�鷽���������غ�ɫ��Һ����
ȡ����
�������Թ��У�����
ʹ���ܽ⣬�ټ���
��Һ���۲�����
����֪���ǰ��U�ι��м����� 100 mLO.5mol��L
-1 CuCl
2��Һ������cʱ��·��һ��ת����0.03mol���ӣ�����������0.64gͭ�����γɵĵͼ������ӵ����ʵ���Ϊ
mol��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�