
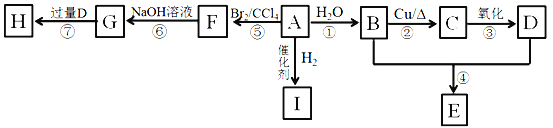
����Ŀ��A��I�dz����л��A������E�ķ���ʽΪC4H8O2��HΪ����ζ����״���ʡ�
��֪��CH3CH2Br��NaOH![]()
��1��0.2mol A��ȫȼ������17.6 g CO2��7.2g H2O����A�Ľṹ��ʽΪ____________��
��2��D�����к��й����ŵ�����Ϊ_____________��
��3���ٵķ�Ӧ����Ϊ____________
��4��G���ܾ��е�����Ϊ__________��
a.���Ʒ�Ӧ b.��NaOH��Һ��Ӧ c.������ˮ
��5����д���ں͢ߵĻ�ѧ����ʽ��
��Ӧ��_________________��
��Ӧ��_________________��
��6��J���л���B��ͬϵ��ұ�B��3��̼ԭ�ӣ�J���ܵĽṹ��___�֣�д�����к�3�������ܵĽṹ��ʽ________________________��
���𰸡� CH2��CH2 �Ȼ� �ӳ� ac 2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O CH2OHCH2OH+2CH3COOH
2CH3CHO+2H2O CH2OHCH2OH+2CH3COOH![]() CH3COOCH2CH2OOCCH3+2H2O 8 (CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3
CH3COOCH2CH2OOCCH3+2H2O 8 (CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3
������������17.6g CO2�����ʵ���Ϊ��n(CO2)=![]() =0.4mol��ˮ�����ʵ���Ϊ
=0.4mol��ˮ�����ʵ���Ϊ![]() =0.4mol��A��һ����������Ԫ���غ��֪0.2mol A��n(C)=0.4mol��n(H)=0.4mol��2=0.8mol����1molA��n(C)=2mol��n(H)=4mol��AΪCH2=CH2��A��ˮ�ӳ�����B��BΪCH3CH2OH����CΪ��ȩ��DΪ���ᣬ�Ҵ������ᷢ��������Ӧ����E��EΪ������������ϩ�������ӳ�����I��IΪ���飻��ϩ����ӳ�����F��FΪ1��2-�������飬 1��2-�������鷢��ˮ������G��GΪ�Ҷ������Ҷ��������ᷴӦ���������Ҷ������ݴ˴��⡣
=0.4mol��A��һ����������Ԫ���غ��֪0.2mol A��n(C)=0.4mol��n(H)=0.4mol��2=0.8mol����1molA��n(C)=2mol��n(H)=4mol��AΪCH2=CH2��A��ˮ�ӳ�����B��BΪCH3CH2OH����CΪ��ȩ��DΪ���ᣬ�Ҵ������ᷢ��������Ӧ����E��EΪ������������ϩ�������ӳ�����I��IΪ���飻��ϩ����ӳ�����F��FΪ1��2-�������飬 1��2-�������鷢��ˮ������G��GΪ�Ҷ������Ҷ��������ᷴӦ���������Ҷ������ݴ˴��⡣
(1)�������Ϸ�����֪AΪ��ϩ��A�Ľṹ��ʽΪCH2=CH2���ʴ�Ϊ��CH2=CH2��
(2)DΪ���Ậ���Ȼ����ʴ�Ϊ���Ȼ���
(3)��Ӧ��ΪCH2=CH2��ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH����Ӧ����ʽΪCH2=CH2+H2O![]() CH3CH2OH���ʴ�Ϊ���ӳɷ�Ӧ��
CH3CH2OH���ʴ�Ϊ���ӳɷ�Ӧ��
(4)GΪCH2OHCH2OH��a��������Ʒ�ӦCH2OHCH2OH+2Na��CH2ONaCH2ONa+H2������a��ȷ��b����������NaOH��Һ��Ӧ����b����c���Ҷ���������ˮ����c��ȷ���ʴ�Ϊ��ac��
(5)��Ӧ�ڣ��Ҵ���Cu��Ag�����������·���������Ӧ����ӦΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ����CH2OHCH2OH+2CH3COOH
2CH3CHO+2H2O����Ӧ����CH2OHCH2OH+2CH3COOH![]() CH3COOCH2CH2OOCCH3+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
CH3COOCH2CH2OOCCH3+2H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O�� CH2OHCH2OH+2CH3COOH
2CH3CHO+2H2O�� CH2OHCH2OH+2CH3COOH![]() CH3COOCH2CH2OOCCH3+2H2O��
CH3COOCH2CH2OOCCH3+2H2O��
(6)BΪCH3CH2OH��J���л���B��ͬϵ��ұ�B��3��̼ԭ�ӣ���JΪ�촼������C5H12��3�ֽṹ��CH3CH2CH2CH2CH3��![]() ��
�� ������CH3CH2CH2CH2CH3����3����ԭ�ӣ�����3�ִ���
������CH3CH2CH2CH2CH3����3����ԭ�ӣ�����3�ִ�������4����ԭ�ӣ�����4�ִ���
 ����1����ԭ�ӣ�����1�ִ������J���ܵĽṹ����8�֣����к�3�����Ľṹ��ʽΪ(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3���ʴ�Ϊ��8��(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3��
����1����ԭ�ӣ�����1�ִ������J���ܵĽṹ����8�֣����к�3�����Ľṹ��ʽΪ(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3���ʴ�Ϊ��8��(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����¯�����з����Ļ�����Ӧ֮һ:FeO(s)+CO(g) ![]() Fe(s)+CO2(g)(����Ӧ����),��ƽ�ⳣ���ɱ�ʾΪ
Fe(s)+CO2(g)(����Ӧ����),��ƽ�ⳣ���ɱ�ʾΪ![]() , ��֪1100��,K=0.263
, ��֪1100��,K=0.263
(1)�¶�����,��ѧƽ���ƶ���ﵽ�µ�ƽ��,��¯��CO2��CO�������ֵ__________(����������С�����䡱),ƽ�ⳣ��Kֵ__________(����������С�����䡱)��
(2)1100 ��ʱ��ø�¯��c(CO2)=0.025 mol��L-1,c(CO)=0.1 mol��L-1,�����������,�÷�Ӧ�Ƿ���ƽ��״̬__________(��ǡ���),��ʱ,��ѧ��Ӧ����v��__________v��(����ڡ���С�ڡ����ڡ�),��ԭ����_______________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ù�ҵұ������ͭ(����Fe2+��AsO2-��Ca2+������)�ᴿ�Ʊ��������ͭ�������������£�

��֪����Fe3+��Cu2+��ʼ������pH�ֱ�2.7��5.4����ȫ������pH�ֱ�Ϊ3.7��6.4��
��Ksp[Cu(OH)2]=2��10-20
��AsO2-+H2O2+H+=H3AsO4�� H3AsO4+Fe3+=FeAsO4��+3H+
(1)�ܽ��������Ҫ���ƺ�ͭ32 g��L-1������ͭ��Һ1.0 L����Ҫ����ұ��������ͭ����������Ϊ___________g��
(2)�ⶨ�ܽ�Һ�е�Fe2+��Ũ�ȣ�����KMnO4����Һ�ζ���ȡ��KMnO4��ҺӦʹ��
________(����ʽ��������ʽ��)�ζ��ܣ����з�Ӧ���ӷ���ʽΪ��______________________����Ҫ�������pH����Һ�е�Fe3+�ѳ����ķ�����___________________________��
(3)��������Ҫ����Һ����ϡ�ͼ�������Һ��pH=5����ϡ�ͺ����Һ��ͭ����Ũ������ܳ���____________mol��L-1��
(4)��������Ҫ�ɷֳ� FeAsO4 ��Fe(OH)3���__________________������Һ����CuSO4��H2O,��Ҫ����________ ��____________�����ˡ�ϴ�ӡ����������
(5)�������ϵ�Ƽ�����ͭ��Ϊ�������Һ������ͭ(��п������������)�Ʊ���ͭ��д�����������ĵ缫��Ӧʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��С������ѪҺ���鵥�У�������Ϊ5.9mmol/L����ʾ�����ָ����������ǣ� ��
A.�ܽ�ȣ�s��
B.���ʵ���Ũ�ȣ�c��
C.����������w��
D.Ħ��������M��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������������Ű��������ӱ��ȵ�������������β����ȼú����ɿ�����Ⱦ��ԭ��֮һ������о���������ķ�Ӧ�������������ͷ��λ�����Ⱦ����Ҫ���塣
(1) ����2NO(g) + 2H2(g) = N2(g) + 2H2O(g) ��H = 665 kJ/mol �ķ�Ӧ��������ɣ�
�� 2NO(g) = N2O2(g) (��)
�� N2O2(g) + H2(g) = N2O(g) + H2O(g) (��)
�� ______________________________ (��)������ɵڢ۲��Ļ�ѧ����ʽ����˾������ܷ�Ӧ���ʵ��ǵ�_____���ķ�Ӧ��(�����)
(2) ��֪��H2(g) + CO2(g) = H2O(g) + CO(g) ��H = + 41 kJ/mol����β���ľ���ԭ����Ҫ���ô�����NO��CO��Ӧת��Ϊ���ֶԴ�������Ⱦ�����壬��д���÷�Ӧ���Ȼ�ѧ����ʽ��______________��
�÷�Ӧ��һ�������´ﵽƽ���Ϊ���ܼӿ췴Ӧ���ʲ��÷�Ӧ���������ƶ����ɲ�ȡ�Ĵ�ʩ�У���__________��
A���ʵ������¶� B���ʵ������¶� C��ѹ���������ѹǿ D��ʹ��������
�÷�Ӧ��ȡ������ʩ���´ﵽƽ���Kֵ��______(����������������С������������)
(3) �����¶����þ�������Ļ�ѧ��Ӧ�ӿ췴Ӧ���ʣ������о�����2NO(g) + O2(g) = 2NO2(g) ��H < 0 ����һЩ������������ij��ѧС��ͨ��ʵ���ò�ͬ�¶��¸÷�Ӧ���ʳ���k (������Ӧ���ʵ�һ������)����ֵ���±���
T(K) | k | T(K) | k | T(K) | k |
143 | 1.48 �� 105 | 273 | 1.04 �� 104 | 514 | 3.00 �� 103 |
195 | 2.58 �� 104 | 333 | 5.50 �� 103 | 613 | 2.80 �� 103 |
254 | 1.30 �� 104 | 414 | 4.00 �� 103 | 663 | 2.50 �� 103 |
��ʵ�����ݲv(��)~c(O2)�Ĺ�ϵ��ͼ��ʾ����x�����ߵ�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬���Ϊ��Ӧ�ĵ�Ϊ_____��(����ĸ)��������ԭ��__________����__________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ۡ��е�ͣ���ԭ����(����)
A. ��ķǽ����Խ��� B. I��I���ļ��ܽ�С
C. �⾧�����ڷ��Ӿ��� D. I��I���ۼ��ļ����ϳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͨˮ���ڹ̻�������������ˮ���Ӽ��ٲ��γɼ�����Һ��������һ������ѧ�ص㣬��ѧ�ҷ����˵綯�Ʒ���ˮ��ij���ʱ�䡣�˷���ԭ����ͼ��ʾ����Ӧ���ܷ���ʽΪ2Cu��Ag2O===Cu2O��2Ag�������й�˵����ȷ���� (����)

A. 2 mol Cu��1 mol Ag2O������������1 mol Cu2O��2 mol Ag���������
B. �����ĵ缫��ӦʽΪ2Cu��2OH����2e��===Cu2O��H2O
C. ����ԭ��ʾ��ͼ�У����������Cu��Ag2O
D. ��ع���ʱ��OH���������ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ������ļס��������أ�����̼�����л���ɫ���������
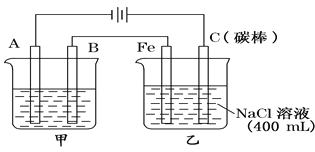
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���A��________�����缫��ӦΪ__________________����һ����1mol��ͭ����ʱ����һ���ܽ��ͭ___________1mol����������������С������������������
��2���ҳ���������������̪��Һ�����һ��ʱ���Fe�缫������________ɫ���缫��ӦʽΪ_________��
��3�����׳��е������ҺΪCuSO4��Һ������������������12��8g�����ҳ��������ų��������ڱ�״���µ����Ϊ_______L,����ʱ�ҳ�ʣ��Һ��Ϊ400 mL���������Һ��pHΪ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com