
����Ŀ��SO2�ĺ����Ǻ���������Ⱦ��һ����Ҫָ�ꡣ��ҵ�ϳ����ô���ԭ�������շ�����SO2��
��1���ڸ�����ִ��������£�CH4��ʹSO2ת��ΪS��ͬʱ����CO2��Һ̬H2O��
��֪��CH4��g��+2O2��g�� = CO2��g��+2H2O��l��= -890.3 kJ/mol
S��s��+O2��g�� = SO2��g�� AH=-291.2 kJ/mol
��CH4��SO2��Ӧ���Ȼ�ѧ����ʽΪ________��
��2���ں����ܱ������У���H2��ԭSO2����S�ķ�Ӧ��������ɣ���ͼ1��ʾ�����ù�����������ʵ����ʵ���Ũ����ʱ��ı仯��ϵ��ͼ2��ʾ��
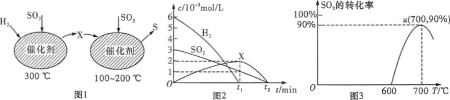
�ٷ�����֪XΪ_____���ѧʽ����0t1ʱ��ε��¶�Ϊ_____.
����H2��ԭSO2����S���ܷ�Ӧ�Ļ�ѧ����ʽΪ_____.
��3����̿����ԭSO2����S2����ѧ����ʽΪ2C��s��+2SO2��g��= S2��g��+2CO2��g�����ں����ܱ������У�1 mol/LSO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3��ʾ��
�ٸ÷�Ӧ����H____������ > ������<����0��
�ڼ���a���ƽ�ⳣ��Ϊ_____.
��4����ҵ����Na2SO3��Һ�������᳧�ķ���SO2��NaHSO3��Һ��
��ij�¶��£���1.0mol/LNa2SO3��Һ���մ�����SO2,����ҺpH����5ʱ���������������½���Ӧ�������ռ�����ʱ��Һ��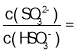 _____������֪���¶���H2SO3�ĵ���ƽ�ⳣ����Ka1=1.50
_____������֪���¶���H2SO3�ĵ���ƽ�ⳣ����Ka1=1.50![]() 10-2,Ka2=1.25
10-2,Ka2=1.25![]() l0-6��
l0-6��
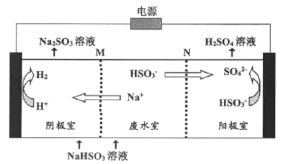
���ö��Ե缫���NaHSO3��ˮ��ʹ����Һ������ԭ������ͼ��ʾ��M��NΪ���ӽ���Ĥ�������ĵ缫��ӦʽΪ��______��
���𰸡�CH4��g����2SO2��g�� = CO2��g����2S��s����2H2O��l�� ��H����295.9kJ/mol H2S 300�� 2H2��SO2 S��2H2O �� 36.45 1/8 HSO3- - 2e- + H2O = SO42- + 3H+
S��2H2O �� 36.45 1/8 HSO3- - 2e- + H2O = SO42- + 3H+
��������
��1��CH4��S��ȼ���ȷֱ�Ϊ890.3kJ/mol��297.2kJ/mol����֪�Ȼ�ѧ����ʽ��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H��890.3kJ/mol�٣�S(s)+O2(g)=SO2(g) ��H��297.2kJ/mol�ڣ����ݸ�˹���ɣ�����-����2�ɵ�CH4(g)+2SO2(g)=CO2(g)+2S(s)+2H2O(l)�ķ�Ӧ�ȣ�
��2���ٸ���ͼ1��֪����300��ʱ��SO2��H2��Ӧ����H2S����100�浽200��ʱ��H2S��SO2��Ӧ����S��ˮ��
�ڸ��ݷ�����֪��SO2��H2���շ�Ӧ����S��ˮ���ݴ�д����ѧ����ʽ��
��3���ٸ���ͼ���֪�������¶ȣ�SO2��ת���ʽ��ͣ�
����������ʽ�������Ӧ����������ƽ��Ũ�ȣ�����ƽ�ⳣ��K= 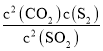 �����㣻
�����㣻
��4���ٸ��ݷ�ӦSO32-+H2O+SO2=2HSO3-��֪����ҺpH����5ʱ����Һ�е�����ΪH2SO3��NaHSO3��c(H+)=10-5mol/L������SO32-+H+HSO3-ƽ�ⳣ��K= 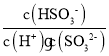 =Ka2=1.25 l0-6��������ã�
=Ka2=1.25 l0-6��������ã�
�ڹ۲�ͼ�ο�֪����������ӷŵ緢����ԭ��ӦΪ�������Ҳ��������ӦΪ������HSO3-ʧȥ���ӱ��SO42-��
��1��CH4��S��ȼ���ȷֱ�Ϊ890.3kJ/mol��297.2kJ/mol����֪�Ȼ�ѧ����ʽ��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H��890.3kJ/mol�٣�S(s)+O2(g)=SO2(g) ��H��297.2kJ/mol�ڣ����ݸ�˹���ɣ�����-����2�ɵ�CH4(g)+2SO2(g)=CO2(g)+2S(s)+2H2O(l)�ķ�Ӧ�ȣ���H��295.9kJ/mol���ʴ�Ϊ��CH4(g)+2SO2(g)=CO2(g)+2S(s)+2H2O(l)��H��295.9kJ/mol��
��2���ٸ���ͼ1��֪����300��ʱ��SO2��H2��Ӧ����H2S����100�浽200��ʱ��H2S��SO2��Ӧ����S��ˮ����0t1ʱ����¶�Ϊ300�棻�ʴ�Ϊ��H2S��300�棻
�ڸ��ݷ�����֪��SO2��H2���շ�Ӧ����S��ˮ���ʻ�ѧ����ʽΪ��2H2��SO2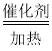 S��2H2O���ʴ�Ϊ�� 2H2��SO2
S��2H2O���ʴ�Ϊ�� 2H2��SO2 S��2H2O
S��2H2O
��3���ٸ���ͼ���֪�������¶ȣ�SO2��ת���ʽ��ͣ���ƽ�����ƣ��˷�ӦΪ���ȷ�Ӧ������H<0���ʴ�Ϊ��<��
�����ڵ�a�Ķ��������ת����Ϊ90%�����У�
2C(s)+2SO2(g)S2(g)+2CO2(g)
��ʼ(c)��1mol/L 0 0
ת��(c)��0.9mol/L 0.45mol/L 0.9mol/L
ƽ��(c)��0.1mol/L 0.45mol/L 0.9mol/L
��ƽ�ⳣ��K= =
=![]() =36.45���ʴ�Ϊ��36.45��
=36.45���ʴ�Ϊ��36.45��
��4�����ݷ�ӦSO32-+H2O+SO2=2HSO3-��֪����ҺpH����5ʱ����Һ�е�����ΪH2SO3��NaHSO3��c(H+)=10-5mol/L������SO32-+H+HSO3-ƽ�ⳣ��K= 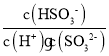 =Ka2=1.25��l0-6�����
=Ka2=1.25��l0-6�����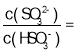 1/8���ʴ�Ϊ1/8��
1/8���ʴ�Ϊ1/8��
��5���۲�ͼ�ο�֪����������ӷŵ緢����ԭ��ӦΪ�������Ҳ��������ӦΪ������HSO3-ʧȥ���ӱ��SO42-���缫����ʽΪ��HSO3- - 2e- + H2O = SO42- + 3H+���ʴ�Ϊ��HSO3- - 2e- + H2O = SO42- + 3H+��


 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʾ��ͼ���Ӧ�ķ�Ӧ�����ȷ������������

A. ��0.01 mol NaOH��0.01 mol Ba��OH��2�Ļ����Һ�л���ͨ��CO2
B. KHCO3��Һ����μ���Ba��OH��2��Һ
C. KAl��SO4��2��Һ����μ���Ba��OH��2��Һ
D. ���������������Ƶ�ƫ��������Һ�еμ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��50 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ��ͼʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
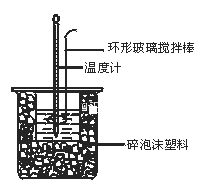
��1��ͼ�л��ν�����ܷ���ͭ����Ʒ����______��ԭ����____________
��2���ձ���������ֽ����������__________________________________
��3��ÿһ��ƽ��ʵ��������Ҫ�۲��¼�����¶���ֵ______
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ_______________���ƫ��ƫС������Ӱ�족����
��5��ʵ���и���60 mL 0.50 mol��L��1�����50 mL 0.55 mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ������к���____________�����ȡ�������ȡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��������һ�ַdz����õĽ�����ͨ������________�С�����Ͷ������ͭ��Һ�У�������Ӧ�����ӷ���ʽΪ_____________��_________________
(2)Na2O2����Ϊ��������еĹ��������乩��ʱ��Ӧ�Ļ�ѧ����ʽ�У�__________��_____
(3)��һ����Һ�����ܺ���Al3����Fe3����K����Mg2����Cu2���������е�һ�ֻ��֡��ּ���Na2O2��ĩ����ɫ��ζ������ų���ͬʱ������ɫ������������Һ�е�ˮ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ�����ƶϣ�

��ԭ��Һ��һ�����е�������__________________��
��һ�������е�������_________________��
�ۿ��ܺ���___________��Ϊ�˽�һ��ȷ�����ܺ��и����ӣ���������ɫ��Ӧ��ʵ�飬����ɫ�ܲ����۲쵽�Ļ������ɫΪ_______ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����C��D��EΪ���ʣ�EΪ���壬FΪ�д��ԵĻ��������֮��������¹�ϵ(��Ӧ�����ɵ�ˮ����Ҫ���������ȥ)��

��1��д���������ʵĻ�ѧʽ��B________��E________��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ������________������Ӧ������________����
��3������Ӧ�����ڼ��������½��У���A��________(�ѧʽ)������Ӧ�����ڳ��������½��У���A��________(�ѧʽ)�������������������µõ���������C���ʣ���Ӧ��ת�Ƶĵ�����֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С������ʵ���ʾ��ͼ��ͼ��ʾ��ͼ�С�������ʾ�������� M��һ�ִ�������������壬YΪ��һ�����壬E���к���ɫ���������ʵ����������ֻ��������������ѡȡ��Na2CO3��Na2O2��NaCl��Na2O��CaCl2��(NH4)2CO3����ʯ�ҵȹ��弰����ˮ���ݴ�ʵ�飬���������գ�

(1)A������װ�õ���Ҫ������ҩƷ��______________________��
(2)B����ѡ�ĸ������________����������______________________________��
(3)C�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��________________________________��
(4)��ȡY�����Dװ�����õ���Ҫ������__________________________��
��ȡY����Ļ�ѧ����ʽ��________________________��
(5)F�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ü�������������ȡ����Ӧ��ȡ����Ʒ����������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС����ʵ������ģ���������̣�����Ƶ�ģ��װ�����£�
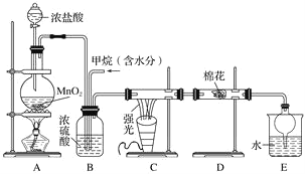
�Իش��������⣺
��1����д��Cװ��������CH3Cl�Ļ�ѧ����ʽ��___________________________________��������ΪE�����Ȼ������������֤������������������ȡ����Ӧ������Ϊ���Ŀ���________(������ȷ����������ȷ��)��
��2��Bװ�������ֹ��ܣ��ٿ����������ʣ��ڻ��Ȼ�����壻��________________��
��3��һ��ʱ�����Dװ���е����ɰ�ɫ��Ϊ��ɫ�������Ͽ���Ԥ�ȵ���________��Һ��
��4��Eװ���г������⣬�����л����E�з�����������ѷ���Ϊ________(����ĸ)��
a����Һ�� b������ c���ᾧ��
��5����1 mol CH4��Cl2����ȡ����Ӧ����ַ�Ӧ�����ɵ�CH3Cl��CH2Cl2��CHCl3��CCl4�����л���������ʵ�����������0.1 mol����μӷ�Ӧ��Cl2�����ʵ���Ϊ________������HCl�������ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�����CaCl2��HCl�Ļ����Һ����μ���Ũ��Ϊ1 mol��L��1��Na2CO3��Һ����Ӧ�����м����Na2CO3��Һ���������������������������ϵ��ͼ��ʾ��
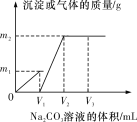
��֪ͼ��V1��V2��1��2��������V2 mL Na2CO3��Һʱ��������Һ��Ũ��Ϊ1 mol��L��1�����Ϊ200 mL����
(1)����V2 mL Na2CO3��Һʱ��������Һ��������________��
(2)ԭ�����Һ��CaCl2��HCl�����ʵ���֮��n(CaCl2)��n(HCl)��________��
(3)m1��________g��m2��________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���絼���Ǻ����������Һ����������С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣��һ���¶��£���0.1mol��L-1KOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol ��L-1������ʹ�����Һ���ζ�������ͼ��ʾ�������й��ж���ȷ����
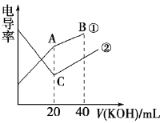
A. �ٱ�ʾ����KOH��Һ�ζ�������Һ
B. A�����Һ����c(CH3COO-)��c(OH-)-c(H+)=0.1 mol��L-1
C. C��ˮ�����c(OH-)����A��ˮ�����c(OH-)
D. A��B��C������Һ����Kw=1.0��10-14
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com