
����Ŀ���Ѽ��仯�����ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á�
(1)��̬��ԭ�ӵļ۵����Ų�ʽΪ_____________������ͬ���ڵ�Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����������ͬ����____________�֡�
(2)�ѱȸ��ᡢ����Ӳ����һ�����˵Ľṹ���ϣ��ѵ�Ӳ�ȱ������ԭ����_________��
(3)��Ũ��TiCl3��������Һ�м������ѣ���ͨ��HCl�����ͣ��ɵõ���λ��Ϊ6�����ΪTiCl3��6H2O����ɫ���壬�þ�����������������ʵ���֮��Ϊ1��5�����������ӵĻ�ѧʽΪ___________��
(4)����Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ľۺϣ���ṹ����ͼ��ʾ��
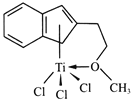
�����M��Ԫ���У��縺��������_________(������)��
��M��̼ԭ�ӵ��ӻ���ʽΪ____________��
��M���________(�����)��
a���м� b���Ҽ� c�����Ӽ� d����λ��
(5)���ʯ(TiO2)�Ǻ��ѵ���Ҫ����֮һ���侧���ṹ(��������ͬλ�õ�ԭ����ͬ)��ͼ��ʾ��
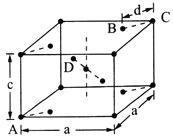
��A��B��C��D 4������������ԭ����________(�����)��
����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����D��ԭ������ΪD(0.19a��____��___)���������ļ���d=______(�ô���ʽ��ʾ)��
���𰸡� 3d24s2 3 Tiԭ�ӵļ۵�������Al�࣬��������ǿ [TiCl(H2O)5]2+ �� sp2 sp3 c BD 0.81a 0.5c ![]()
�����������������(1)��ԭ�Ӻ�����22�����ӣ����ݺ�������Ų�����д��̬��ԭ�ӵļ۵����Ų�ʽ����̬ԭ�ӵ�δ�ɶԵ�����Ϊ2������������δ�ɶԵ�����Ϊ2��Ԫ����Ge��Se��Ni����3����(2) Tiԭ�ӵļ۵�������4����ԭ�ӵļ۵�������3��(3).��λ��Ϊ6��������������ʵ���֮��Ϊ1��5��������������1����ԭ�ӡ�5��ˮ���ӣ�(4) �����M��Ԫ����Ti��C��H��O��Cl���ǽ�����Խǿ�縺��Խ��M����˫��̼�͵���̼ԭ���������۵���Ϊ�Ҽ���˫����1����������1��������������M�Ľṹͼ��������λ����(5) �ٸ��ݾ�̯ԭ�����й���ԭ��![]() ����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2�����������ݾ����ṹ����Dԭ�����ꣻ����ͼʾ��
����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2�����������ݾ����ṹ����Dԭ�����ꣻ����ͼʾ��![]()
������(1)��ԭ�Ӻ�����22�����ӣ���̬��ԭ�ӵļ۵����Ų�ʽΪ3d24s2����̬��ԭ�ӵ�δ�ɶԵ�����Ϊ2������������δ�ɶԵ�����Ϊ2��Ԫ�ػ���3�֣��ֱ���Ge��Se��Ni�� (2) Tiԭ�ӵļ۵�������4����ԭ�ӵļ۵�������3��Tiԭ�ӵļ۵�������Al�࣬��������ǿ�������ѵ�Ӳ�ȱ�������(3).��λ��Ϊ6��������������ʵ���֮��Ϊ1��5��������������1����ԭ�ӡ�5��ˮ���ӣ����Ը�������ӵĻ�ѧʽΪ[TiCl(H2O)5]2+��(4) �����M��Ԫ����Ti��C��H��O��Cl������O�ķǽ�������ǿ���ǽ�����Խǿ�縺��Խ�����Ե縺��������������M����˫��̼�͵���̼ԭ������������M��̼ԭ�ӵ��ӻ���ʽΪsp2�� sp3���۵���Ϊ�Ҽ���˫����1����������1��������������M�Ľṹͼ��������λ����û�����Ӽ�����ѡc��(5) �ٸ��ݾ�̯ԭ�����й���ԭ��![]() ����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2����֪��ԭ����BD�������ݾ����ṹ����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����Dԭ��������(0.19a��0.81a��0.5c)������ͼʾ��
����������ͬλ�õ�ԭ����ͬ����������ԭ�ӱ���1:2����֪��ԭ����BD�������ݾ����ṹ����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����Dԭ��������(0.19a��0.81a��0.5c)������ͼʾ��![]() ����d=
����d=![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����е�����ԭ���ӻ������������ͬ����( )
A. CO2��SO2 B. CH4��NH3 C. BeCl2��BF3 D. C2H2��C2H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ľ����ά�ɺϳ�ҩ���м���E,���ܺϳɸ߷��ӻ�����H,�ϳ�·������:
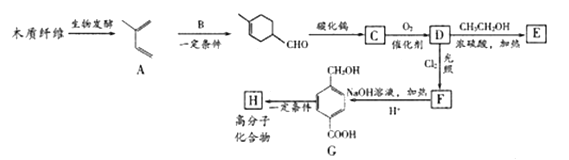
��֪:
��
��![]()
�ش���������:
��1��C�Ļ�ѧ������__________,F���������ŵ�������__________��
��2��G![]() H�ķ�Ӧ������________��
H�ķ�Ӧ������________��
��3��B�Ľṹ��ʽ��____________
��4��F��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��___________
��5����������������E��ͬ���칹�干��_____�֡�
�ٷ����б�������������λȡ����
���ܷ���������Ӧ
���ܺ��Ʒ�Ӧ�ų�����������FeCl3��Һ������ɫ��Ӧ
��6�����������Ϣ��д����2-��ϩ,HOOCCH=CHCOOHΪԭ��,�Ʊ� �ĺϳ�·��:________________(�����Լ���ѡ)��
�ĺϳ�·��:________________(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ӵ��γɹ���ʾ��ͼ(����ͼ)�ش����⣺

(1)H��H���ļ���Ϊ________���١����У���ϵ�����ɸߵ��͵�˳����________��
(2)����˵������ȷ����( )
A�����������к���һ���м�
B���ɢٵ��ܣ������ں˼���ֵļ�������
C���ɢܵ��ݣ�����������������
D�����������к���һ�����Թ��ۼ�
(3)���ֳ�����ѧ���ļ������±���
��ѧ�� | Si��O | H��O | O==O | Si��Si | Si��C |
����/kJ��mol��1 | 460 | 464 | 498 | 176 | x |
��ش��������⣺
�ٱȽ�Si��Si����Si��C���ļ��ܴ�С��x________(�>������<������)176��
��H2����Ϊ21���������������ȼ�ϣ������п�ѧ��������ǡ�21���͵���Դ������δ����ʯ�͡��Ĺ۵㡣�Լ��㣺ÿǧ��H2ȼ��(����ˮ����)�ų�������ԼΪ________��ÿĦ������ȫȼ�շų�������ԼΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
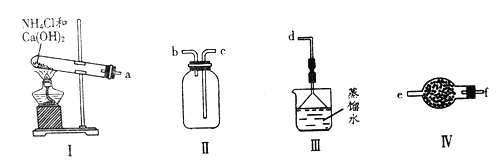
����Ŀ��ij��ѧʵ��С������ͼװ����ȡ���ռ���������İ�������̽���������й����ʡ�
�ش���������:
��1��д������װ��I��ȡ�����Ļ�ѧ����ʽ_________________________________��
��2���������������������ӵ�˳��:a��___________________________��d(����ĸ��ű�ʾ)��
��3��ʵ����װ��III������________________��
��4��װ��IV������������Ϊ________��ʢװ���Լ�Ϊ______________��
��5���ڼס��Ҳ��������зֱ��ռ��������Ȼ��⣬����ͼװ�ý���ʵ�顣������K,�۲쵽��ʵ��������_____________________________��
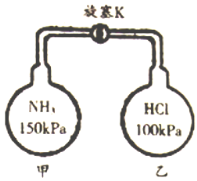
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������з�Ӧ�У�ˮ����������������ԭ������(����)
A. 2F2��2H2O===4HF��O2 B. 2Na��2H2O===2NaOH��H2��
C. CaO��H2O===Ca(OH)2 D. 2H2O![]() 2H2����O2��
2H2����O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���������������ƹ�������1.0 mol��L��1������������Һ240 mL��
��1��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ������ڼ��㡡���ܽ⡡��ҡ�ȡ���ת�ơ���ϴ�ӡ��߶��ݡ�����ȴ����ҡ��
����ȷ�IJ���˳��Ϊ__________������ţ�����ʵ������õ�����������ƽ��ҩ�ס����������ձ���____________________��
��2��ijͬѧ�������������Ƶ�������������������ƽ�����ձ�����������ƽƽ����״̬����ͼ��ʾ���ձ���ʵ������Ϊ__________g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�____________g �������ơ�
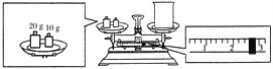
��3��ʹ������ƿǰ������е�һ��������__________��
��4�������ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���_________������ĸ����
A��ת����Һʱ������������������ƿ����
B������ʱ���ӿ̶���
C��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
D�����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�������ĺ���������Ҫ�����������������ʼ����ǵ���������в��ֲ�������ͼ�����ʡ�Ϊ�˴Ӻ��������л���������������µĹ�������:
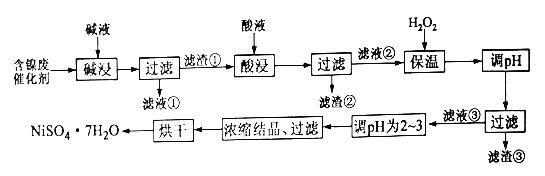
������������ȫ����ʱ��Һ��pH����:
������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
pH | 5.2 | 3.2 | 9.7 | 9.2 |
�ش���������:
(1)�����Ŀ����Ϊ�˳�ȥ____________
(2)ijѧϰС����ʵ������ģ����������,����ϴ�������ٵIJ���_________
(3)����H2O2������һ��ʱ������У����µ��¶Ȳ��˹��ߵ�ԭ��Ϊ______,����H2O2������Ӧ�����ӷ���ʽΪ___________������H2O2���º��pH,���з�Χ��������______(��ѡ�����)��
A.1.8~3.2 B.2.0~3.0 C.3.3~5.2 D.9.2~9.7
(4)�����۵���Ҫ�ɷ�Ϊ_______,��Һ�۵�pHΪ2~3��Ŀ��Ϊ________
(5)Ϊ�ⶨ��Ʒ��NiSO4��7H2O���ȣ�ijͬѧ��һ��������Ʒ����ˮ,�������м���������BaCl2��Һ,���ˡ�ϴ�ӳ���������,����������ͨ�����㷢�ֲ�Ʒ��NiSO4��7H2O��������������100%,����ܵ�ԭ��Ϊ____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����NaB(OH)4��Һ�Ʊ�H3BO3��ԭ������ͼ��ʾ�����������������

A. M�ҷ����ĵ缫��Ӧʽ��2H2O-4e-=O2��+4H+
B. a��cΪ�����ӽ���Ĥ��bΪ�����ӽ���Ĥ
C. N����a��<b��
D. ������ÿ����1 mol H3BO3�������ҹ�������״����16.8 L����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com