
����Ŀ���������ڼ�չ��տɴ����ȷ������������ĸ�����Al��Al2O3��Cr2O3�ȣ��н�����������Ϊʵ�ָ��������������á��乤���������£�

��1�����ȷ�ұ�����������������˽�������__________��������ԡ���ԭ�ԡ�����
��2����Һ1�е���������CrO42-��__________��
��3������I����Cr2O3����ķ�Ӧ�У�������0.4molCrO42-�����������������ʵ�����__________��
��4��ͨ��CO2������ҺpHʵ�����ʵķ��롣
������A���յõ�Al2O3�����õ�ⷨұ��Al��ұ��Al�Ļ�ѧ����ʽ��____________________��
������B���ȷֽ��������ʵ�ѭ�����ã�B��__________��
����֪��2CrO42-+2H+![]() Cr2O72-+H2O K=4.0��1014
Cr2O72-+H2O K=4.0��1014
��Һ3��Cr2O72-���ܶ���0.04mol/L����CrO42-��Ũ����__________mol/L��
��5������II��Ŀ���ǵõ�K2Cr2O7��Ʒ����ͬ�¶��»�������ܽ�ȣ�g/100gH2O��
���������� | 0�� | 20�� | 40�� | 60�� | 80�� |
NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 |
K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 |
K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 |
Na2Cr2O7 | 163 | 183 | 215 | 269 | 376 |
��ϱ������ݷ���������II�IJ����ǣ�____________________�����ˣ��õ�K2Cr2O7��Ʒ��
���𰸡� ��ԭ�� AlO2-��OH- 0.3mol 2Al2O3�����ڣ�![]() 3O2��+4Al NaHCO3 0.01 ����Һ���м���ϡ�����K2SO4���������Ũ�������½ᾧ
3O2��+4Al NaHCO3 0.01 ����Һ���м���ϡ�����K2SO4���������Ũ�������½ᾧ
����������1�����ȷ�ұ�����������������˽������Ļ�ԭ������2��Al��Al2O3��Cr2O3��NaOH��Ӧ��������Na2CrO4������ƫ�����ƺ�����NaOH����Һ1�е���������CrO42-������AlO2-��OH-����3����Ӧ�����������������ɵ�ʧ�����غ��֪��0.4mol��(6-3)=n(O2) ��4��n(O2)=0.3mol����4���ٵ�ⷨұ��Al�Ļ�ѧ����ʽ�ǣ�2Al2O3�����ڣ�![]() 3O2��+4Al������ƫ��������Һ��ͨ�������̼��������̼���ƻ�̼�����ƣ�����̼�����������ȷֽ⣬��B��̼�����ơ����ɴ˷�Ӧƽ�����ʽ��֪��k= c(Cr2O72-)/c2(CrO42-)c2(H+)=4.0��1014������c(Cr2O72-)=0.04mol/L��c(H+)=10-6 mol/L��c(CrO42-)==0.01 mol/L����5��Ҫ�õ������K2Cr2O7��Ʒ������������ᣬ�ٽ�2CrO42-+2H+
3O2��+4Al������ƫ��������Һ��ͨ�������̼��������̼���ƻ�̼�����ƣ�����̼�����������ȷֽ⣬��B��̼�����ơ����ɴ˷�Ӧƽ�����ʽ��֪��k= c(Cr2O72-)/c2(CrO42-)c2(H+)=4.0��1014������c(Cr2O72-)=0.04mol/L��c(H+)=10-6 mol/L��c(CrO42-)==0.01 mol/L����5��Ҫ�õ������K2Cr2O7��Ʒ������������ᣬ�ٽ�2CrO42-+2H+![]() Cr2O72-+H2O�����ƶ���ͬʱ����K2Cr2O7���ܽ�����¶ȱ仯�ϴ����ɲ�ȡ���½ᾧ�����������������Һ���м���ϡ�����K2SO4���������Ũ�������½ᾧ��
Cr2O72-+H2O�����ƶ���ͬʱ����K2Cr2O7���ܽ�����¶ȱ仯�ϴ����ɲ�ȡ���½ᾧ�����������������Һ���м���ϡ�����K2SO4���������Ũ�������½ᾧ��


 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ж��ֺ����ᣬ�ڹ�ҵ���й㷺��Ӧ�ã�������ͬ��Ҳ����Ҫ�Ļ�����Ʒ��
��.���������( Na2S2O3)�����������Լ������ﻹԭ���������ȡ������ֽ⡣��ҵ�ϳ����ú����ˮ����Na2S2O3��5H2O��ʵ���ҿ�������װ��ģ�����ɹ��̣�
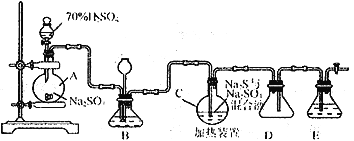
��ƿC�з�����Ӧ������
Na2S(aq)+H2O(l)+SO2(g)==NaSO3(aq)+H2S(aq)������
2H2S(aq)+SO2(g)=3S(s)+2H2O(l)������
S(s)+Na2SO3(aq) ![]() Na2S2O3(aq)������
Na2S2O3(aq)������
��1����ƿA�з�����Ӧ�����ӷ���ʽΪ_________________________________��װ��D��������__________________________��
��2��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��_____________��
a.����ˮ b.����Na2SO3��Һ c.����NaHSO3��Һ d.����NaHCO3��Һ
��3����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ��������������______
a.�ձ� b.��� c.�Թ� d.��ƿ
��4��Ϊ�˱�֤Na2S2O3�IJ�����ʵ����ͨ��SO2���ܹ�����ԭ����_____________________��
��.����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��(Na2S2O5����ˮ������NaHSO3��
��5��֤��NaHSO3��Һ��HSO3-����̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________________��
a.�ⶨ��Һ��pH b.����Ba(OH)2��Һ c. .��������
d.����Ʒ����Һ e.����ɫʯ����ֽ���
��6�����ѾƳ���Na2S2O5�������������ⶨ�����Ѿ��п��������IJ�������������SO2�������ķ�����������֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2+I2+2H2O=H2SO4+2HI����
![]()
������������ʵ�飬���ı�I2��Һ25.00mL���ô�ʵ������Ʒ�п��������IJ�������������SO2������Ϊ______g/L
��������ʵ������У�HI���ܻᱻ������������Ӧ�Ļ�ѧ����ʽΪ_____________________________�����в���HI�������������ᵼ�²�õĽ��_______������ƫ������ ƫ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����в��ֶ���������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | M���ϵ�������K���ϵ�����3�� |
X | �����������Ǵ�����������2�� |
Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
Z | Ԫ�����������+7�� |
(1)Ԫ��X��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����____��
(2)Ԫ��Y����Ԫ���γ�һ������YH4+��д�������ĵ���ʽ____����Ԫ�ط��ű�ʾ����
(3)Ԫ��Z��Ԫ��T��ȣ��ǽ����Խ�ǿ����____����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����____��
a��������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b��Z���⻯���T���⻯���ȶ�
c��һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
(4)T��һ����������ʹZ���ʵ�ˮ��Һ��ɫ��Ӧ�����ӷ���ʽΪ______��
(5)T�����ڱ��е�λ���ǵ�________���ڣ���_________�塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й���������ʵ�˵������ȷ���ǣ� ��
A. ���������Ҫͨ����ܷ�������
B. ������Һ�ﵽ����ƽ��ʱ��������CH3COO��+H+![]() CH3COOH
CH3COOH
C. H2SO4�ǹ��ۻ������������������ʣ�NaOH�����ӻ������������ǿ�����
D. ���������Һ�У��������ʷ��ӣ��������ʵ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���V1 L 0.1mol/L NaOH��Һ��V2 L 0.6mol/L NaOH��Һ��ϣ�������ҺŨ��Ϊ0.3mol/L�������Ϻ�����Ϊ���ǰ������ͣ�V1��V2=�� ��
A.1��2
B.2��1
C.3��2
D.2��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ѧ�ҷ�����һ�����Ϳɿص�ء����ˮ���,����ԭ����ͼ��ʾ�������й�˵������ȷ����(����)

A. ʯī�������ķ�Ӧ��2H2O+2e�� === H2��+2OH��
B. �л�����ʺ�ˮ��Һ�����Ի�������
C. ��װ�ò������ṩ����,���ɵõ���������
D. ��״���²���22.4 L������ʱ,��������﮵�����Ϊ14 g
���𰸡�D
��������A������ͼʾ��Ϣ��֪,ʯī�缫�ϲ���H2����ʯī�缫Ϊ�������缫��ӦΪ��2H2O+2e=H2��+2OH����A��ȷ��B��Li���л�����ʲ���Ӧ����Li��ˮ��Ӧ����Li��ˮ�Ӵ������в��ֻ�ѧ��ֱ��ת��Ϊ���ܣ������л�����ʺ�ˮ��Һ�����Ի�����B��ȷ��C����ԭ����ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�װ�ò������ṩ���ܣ����ҷ�Ӧ���������������ṩ����Դ����C��ȷ��D��������Ǹ�������D��������ѡD��
�����͡���ѡ��
��������
26
����Ŀ��һ�����͵ġ��-����CO2��ء����ṹ��ͼ��ʾ������˵������ȷ����

A. ��װ���ǻ�ѧ��ת��Ϊ����
B. ���øü����ɼ�����������CO2���ŷ�
C. �����ĵ缫��ӦΪ��2CO2 + 2e- = C2O42-
D. ÿ����10.2g Li2C2O4����0.2mol Li+������Ǩ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ÿ������ͻ���ִ����������ܼ��Ȳ���10�ף���ʹ������ʻ���裬���й�������˵����ȷ���ǣ� ��
A.��ȷ������ˮ�ε�ֱ����С
B.����������������
C.����ˮ�ε�ֱ����1nm��100nm��
D.����ˮ�ε�ֱ��С��1nm
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��500mLKNO3��Cu(NO3)2�Ļ����Һ�У�c��NO3-��=6.0mol/L����ʯī���缫������Һ����ͨ��һ��ʱ����������ռ���22.4L���壨��״���������������Һ�������Ϊ500mL������˵����ȷ���ǣ�������
A. ԭ�����Һ��K+�����ʵ���Ũ��Ϊ1mol/L
B. �����������й�ת��4mol����
C. ����CuO��ʹ��Һ�ָ���ԭ���ijɷֺ�Ũ��
D. ������Һ��H+�����ʵ���Ũ��Ϊ2mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����0.50 mol��L��1 NaCl��Һ480 mL�������в������������ʵ������֣�ʹ��������������
��1��ѡ����������ɱ�ʵ������õ��������У�������ƽ�������룬��С����Ϊ5g������Ͳ��ҩ�ס��ձ�����������____________��____________�Լ�����������Ƭ��ֽ��
��2�����㡣���Ƹ���Һ��ȡNaCl����____________g��
��3��������
����ƽ��ƽ֮��Ӧ����ƽ���������ij��λ�ã�������ͼ����һ�����߱���������Ե������λ�ã�
![]()
������������NaClƷ��Ӧ������ƽ��____________��������������������������
��������ϣ���ҩƷ�����ձ��С�
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����________________________��
��5����Һ��ϴ�ӡ�����ҺʱӦʹ��________________________��������Ҫϴ���ձ�2��3����Ϊ��____________________________________��
��6�����ݡ�������ƿ�м�ˮ��Һ��ӽ��̶���____________��������____________��ˮ��ʹ��Һ��Һ����̶������С�
��7��ҡ�ȡ�װƿ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com