
| ʵ���� | NaOH��Һ��Ũ�� ��mol/L�� | ����NaOH��Һ�� �����mL�� | ����������Һ����� ��mL�� |
| 1 | 0.10 | 19.98 | 20.00 |
| 2 | 0.10 | 20.02 | 20.00 |
| 3 | 0.10 | 20.00 | 20.00 |
| c(��)��V(��) |
| V(��) |
| V(��)��c(��) |
| V(����) |
| 19.98+20.02+20.00 |
| 3 |
| c(��)��V(��) |
| V(��) |
| 0.10mol/L��20.00mL |
| 20.00mL |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
18 8 |
1 1 |
2 1 |
17 8 |
1 1 |
18 8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������ԭ�ӹ�ƽ�� |
| B����ȩ�����Ͷ�����̼��̼ԭ�Ӿ�����sp2�ӻ� |
| C������������̼�ǷǼ��Է��ӣ�ˮ�ͼ�ȩ�Ǽ��Է��� |
| D��ˮ�ķе�ȼ�ȩ�ߵö࣬����Ϊˮ���Ӽ����γ����������ȩ���Ӽ䲻���γ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������������õ���չ�ԣ�����Ϊ���������е�ԭ�Ӳ��ڻ��������н�����δ�ƻ� |
| B��������ͼ���кڵ��������ָ�������Ķ��� |
| C�����м��Լ��ķ���һ���Ǽ��Է��� |
| D������Խ��ʾ�÷���Խ�������ȷֽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

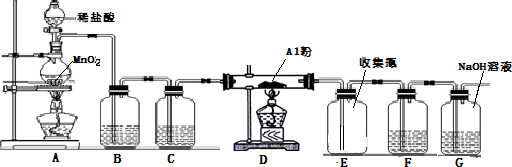
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com