
����Ŀ��������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ����ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ�⡣�������ѧ֪ʶ�ش��������⣺
(1)AlCl3��Һ��______��(��ᡱ���С����)ԭ����_________________________(�����ӷ���ʽ��ʾ)������AlCl3��Һ���ɣ����գ������Ҫ�õ����������________������AlCl3��Һ��NaHCO3��Һ��ϣ��÷�Ӧ�����ӷ���ʽΪ__________________________________________��
(2)��1L0.2 mol��L��1HA��Һ��1L0.1 mol��L��1NaOH��Һ��������(��Ϻ���Һ����仯���Բ���)����û����Һ��c(Na��)>c(A��)����
�ٻ����Һ�У�c(A��)________c(HA)(�>����<����������ͬ)��
�ڻ����Һ�У�c(HA)��c(A��)________0.1 mol��L��1��
(3)�����£���NaOH��Һ��c(OH��)��NH4Cl��Һ��c(H+)��ͬ���ֽ�NaOH��Һ��NH4Cl��Һ�ֱ�ϡ��10����ϡ�ͺ�NaOH��Һ��NH4CI��Һ��pH�ֱ���pH1��pH2��ʾ����pH1 +pH2________(�>����<������)14��
(4)pH��ͬ�Ģ�CH3COONa����NaHCO3����NaClO������Һ��c(Na��)��___________________��
���𰸡��� Al3����3H2O![]() Al(OH)3��3H�� Al2O3 Al3����3HCO3����Al(OH)3����3CO2�� �� �� �� �٣��ڣ���
Al(OH)3��3H�� Al2O3 Al3����3HCO3����Al(OH)3����3CO2�� �� �� �� �٣��ڣ���
��������
��1�������Ȼ���ˮ���Լ����������ˮ��ƽ���Ӱ����������ݷ�Ӧ�����������д����ʽ��
��2�����ݵ���غ�������غ�����жϣ�
��3������ϡ�����ж�ˮ��ƽ���Ӱ������жϣ�
��4��������Խ������Ӧ�����Խ����ˮ�������
��1���Ȼ�����Һ��������ˮ�⣬��Һ�����ԣ�ˮ�ⷽ��ʽΪAl3����3H2O![]() Al(OH)3��3H����ˮ�����ȣ����ȴٽ�ˮ�⣬���ɵ�HCl�ӷ���������ɵõ���������������ʱ���������ֽ�������������ˮ�����������Ҫ�õ����������Al2O3������AlCl3��Һ��NaHCO3��Һ��϶���ˮ����ٽ��������������Ͷ�����̼���÷�Ӧ�����ӷ���ʽΪAl3����3HCO3����Al(OH)3����3CO2����
Al(OH)3��3H����ˮ�����ȣ����ȴٽ�ˮ�⣬���ɵ�HCl�ӷ���������ɵõ���������������ʱ���������ֽ�������������ˮ�����������Ҫ�õ����������Al2O3������AlCl3��Һ��NaHCO3��Һ��϶���ˮ����ٽ��������������Ͷ�����̼���÷�Ӧ�����ӷ���ʽΪAl3����3HCO3����Al(OH)3����3CO2����
��2���ٽ�1L0.2 mol��L��1HA��Һ��1L0.1 mol��L��1NaOH��Һ��������(��Ϻ���Һ����仯���Բ���)��������Һ�������ǵ�Ũ�ȵ�HA��NaA�Ļ����Һ����û����Һ��c(Na��)��c(A��)������ݵ���غ�c(Na��)+c(H��)��c(A��)+c(OH��)��֪c(H��)��c(OH��)������Һ�Լ��ԣ���˵��ˮ��̶ȴ��ڵ���̶ȣ����Ի����Һ�У�c(A��)��c(HA)��
��������Һ�������ǵ�Ũ�ȵ�HA��NaA�Ļ����Һ��Ũ�Ⱦ���0.05mol/L������������غ��֪�����Һ�У�c(HA)��c(A��)��0.1 mol��L��1��
��3����ϡ��ǰNaOH��Һ��NH4Cl��Һ��pH�ֱ���pH3��pH4��ʾ����NaOH��Һϡ��10������������������һԪǿ���pH1��pH3��1���Ȼ����ǿ�������Σ�笠�ˮ�⣬ϡ�ʹٽ�笠�ˮ�⣬��ϡ��10����pH2��pH4+1������NaOH��Һ��c(OH��)��NH4Cl��Һ��c(H+)��ͬ����pH3+pH4��14������pH1 +pH2��pH3��1+pH4+1��14��
��4����������ӵ�ˮ��̶�Խ����ͬpH��������Һ��Ũ��ԽС��������Ũ��ԽС������ˮ��̶Ȣ٣��ڣ��ۣ���pH��ͬ�Ģ�CH3COONa����NaHCO3����NaClO������Һ��c(Na��)Ϊ�٣��ڣ��ۡ�


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհס�
(1)�ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��_______��ֱ�������һ���������Һ��________ɫ��Ϊ________ɫ����________Ϊֹ��
(2)���в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���__________��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
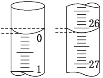
(3)���ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ_______mL���յ����Ϊ_______mL������������Һ�����Ϊ_______mL��
(4)ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ� ���� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1 ��������/mL | ||
�ζ�ǰ���� | �ζ������ | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ϱ�������ʽ�����NaOH��Һ�����ʵ���Ũ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�������Ա��Zn�ͼ⾧ʯ������п��ZnMn2O4��Ϊ�缫���������Ƴ�һ��ˮϵп���ӵ�ء��õ�ص��ܷ�Ӧ����ʽ��xZn + Zn1xMn2O4![]() ZnMn2O4��0 < x < 1��������˵����ȷ����
ZnMn2O4��0 < x < 1��������˵����ȷ����
A. ���ʱ��Zn2+��ZnMn2O4�缫Ǩ��
B. ���ʱ��������Ӧ��ZnMn2O4 2xe����Zn1-xMn2O4+xZn2+
C. �ŵ�ʱ��ÿת��1mol e-��ZnMn2O4�缫��������65g
D. ��ŵ�����У�ֻ��ZnԪ�صĻ��ϼ۷����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ƽ����ʽ KMnO4+ HCl(Ũ)�� KCl+ MnCl2+ Cl2��+ H2O������˫�߷������˷�Ӧ��___��
��2��ָ��___����������___Ԫ�ر�����������������___���˷�Ӧ�У�HCl���ֵ�������___�Ժ�___�ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ������Cr(��)�Ĵ�������������ͼ��
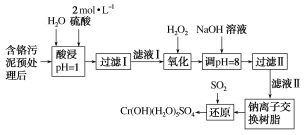
��֪���������ȡҺ�еĽ���������Ҫ��Cr3���������Fe3����Al3����Ca2����Mg2����
�ڳ����£�����������������������ʽ����ʱ��Һ��pH�����
��Cr(OH)(H2O)5SO4�������
������ | Fe3�� | Mg2�� | Al3�� | Cr3�� | |
������ȫʱ��pH | 3.7 | 11.1 | 5.4(��8�ܽ�) | 9(��9)�ܽ� |
��1��ʵ������18.4mol��L��1��Ũ��������480 mL2mol��L��1�����ᣬ��Ҫ��ȡŨ����___mL������ʱ���ò�����������Ͳ���ձ��Ͳ������⣬����____��
��2��H2O2�������ǽ���Һ���е�Cr3��ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��_____��
��3������NaOH��Һʹ��Һ�ʼ��ԣ��ȿ��Գ�ȥijЩ�������ӣ�ͬʱ�ֿ��Խ�Cr2O72-ת��Ϊ___(�����Ļ�ѧʽ)��
��4�������ӽ�����֬�ķ�Ӧԭ��ΪMn����nNaR=MRn��nNa���������������ӽ�����֬�ɳ�ȥ��Һ���еĽ�����������___��
��5��д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ��__��
��6�������ζ����Dzⶨ����Ũ�ȵķ���֮һ��Ϊ�˲ⶨij��ˮ��SCN����Ũ�ȣ����ñ�AgNO3��Һ�ζ�����Һ����֪��
�������� | AgCl | AgI | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | �� | �� | ש�� | �� |
Ksp | 1.8��10��10 | 8.3��10��17 | 1.2��10��16 | 3.5��10��11 | 1.0��10��12 |
�ζ�ʱ��ѡΪ�ζ�ָʾ������___(����)���ζ��յ��������___��
A��NaCl B��K2CrO4 C��KI D��NaCN
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ǻ�ѧ�о��Ļ��������й��ڸ�ʵ��װ�õ�������ȷ������ ��

A. װ�âٳ����ڷ��뻥�����ܵ�Һ������
B. װ�âڿ���������NH3��HCl���壬����ֹ����
C. װ�âܿ����ڸ���ռ��Ȼ��⣬�����ն�����Ȼ���
D. װ�âۿ������ռ�H2��CO2��Cl2��NH3������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʮ�����ʣ���Һ̬HCl ��NaHCO3 ��NaCl��Һ ��CO2 �����Ǿ��� ��Ba(OH)2 �ߺ��ɫ�������������� ��NH3H2O ����� ��Al2(SO4)3
��1������ʮ��������������������ˮ��Һ�пɷ�����Ӧ�����ӷ���ʽΪ��H++OH��=H2O���÷�Ӧ�Ļ�ѧ����ʽΪ______________________________________��
��2������ˮ�еĵ��뷽��ʽΪ______________________________��
��3��θҺ�к������ᣬθ�������˳���θ�����ĵĸо���������ˮ������������С�մ�NaHCO3����������θ����࣬��д���䷴Ӧ�����ӷ���ʽ_________________���������ͬʱ��θ����Ϊ��θ�ڴ��ף����ܷ���С�մ�ʱ����ú�Al(OH)3��θҩ����θ��ƽ��������θ�ᷴӦ�����ӷ���ʽ________��
��4��д�����з�Ӧ�����ӷ���ʽ��
��Ba(OH)2��Һ����μ���������Һ___________________________��
��Ba(OH)2��Һ��ͨ�������CO2_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D���ֽ������±���װ�ý���ʵ�飬����ʵ�����������
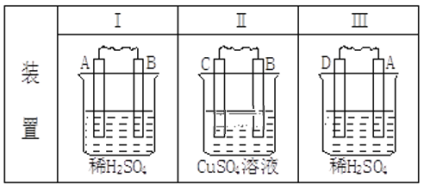
���� | ��I������A���ܽ� | ����C���������� | ����A����������� |
��������Ӧʽ | ___ | ___ | |
���ֽ��������ǿ������˳��___�� | |||
��װ�ã�����A�������������ڱ�״���µ����Ϊ224mL����ͨ�������еĵ��ӵ����ʵ���Ϊ___mol�� | |||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ28g������Ͷ������ϡ��������ȫ�ܽ⣬
��1�������������������
��2������״�����μӷ�Ӧ���������ʵ����Ƕ��٣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com