
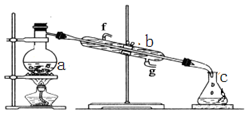
���� ��1����ͼ��֪���������ƣ�
��2����ˮ�½��ϳ�������������ͣ��ʱ�䳤����ȴЧ���ã�
��3���������Ȼ�̼�;ƾ��Ļ�����ȡ������Ҫ�¶ȼƲⶨ�¶ȣ�
��4������װ��������ˮ������ʱ��ֹҺ����ҷ��ڣ�
��5��������0.1mol•L-1NaOH��Һ480mL��ѡ��500mL����ƿ�����ձ����ܽ⡢��ȴ��ת�Ƶ�����ƿ�ж��ݣ�
�ڽ��m=cVM���㣻
��������Һ�IJ���Ϊ���㡢�������ܽ⡢��ȴ��ϴ�ӡ�ת�ơ����ݡ�ҡ�ȡ�װƿ��
�ܽ��c=$\frac{n}{V}$��֪��������������nƫ���VƫС���ᵼ��������Һ��Ũ��ƫ���Դ������
��� �⣺��1������a��b�����Ʒֱ�Ϊ������ƿ�������ܣ��ʴ�Ϊ��������ƿ�������ܣ�
��2��ʵ������У���Ҫͨ��ˮ��ͼ�еĽ�ˮ������g �����ʴ�Ϊ��g��
�������Ȼ�̼�;ƾ��Ļ�����ȡ������Ҫ�¶ȼƲⶨ�¶ȣ�ͼ��ȱ�ٵ�����Ϊ�¶ȼƣ��ʴ�Ϊ���¶ȼƣ�
��4������װ��������ˮ������ʱ��ֹҺ����ҷ��ڣ���ʵ��ʱa�г�������������ˮ�⣬��������������Ƭ����ʯ�����������Ƿ�ֹ���У��ʴ�Ϊ����
��5��������0.1mol•L-1NaOH��Һ480mL��ѡ��500mL����ƿ�����ձ����ܽ⡢��ȴ��ת�Ƶ�����ƿ�ж��ݣ�����Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܡ�500mL����ƿ���ʴ�Ϊ��500mL����ƿ��
��ʵ��ʱ��Ҫ����������������Ϊ0.5L��0.1mol/L��40g/mol=2.0g���ʴ�Ϊ��2.0��?
��������Һ�IJ���Ϊ���㡢�������ܽ⡢��ȴ��ϴ�ӡ�ת�ơ����ݡ�ҡ�ȡ�װƿ������ȷ�IJ���˳����BCAFED���ʴ�Ϊ��BCAFED��
��A������ʱ������������룬��������ƫ��nƫ����Ũ��ƫ��ѡ��
B��δϴ���ܽ�NaOH���ձ���nƫС��Ũ��ƫС���ʲ�ѡ��
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У�VƫС����Ũ��ƫ��ѡ��
D������ƿδ���T����������Һ����ʵ����Ӱ�죬�ʲ�ѡ��
E������ʱ���ӿ̶��ߣ�Vƫ����Ũ��ƫС���ʲ�ѡ��
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�Vƫ����Ũ��ƫС���ʲ�ѡ��
�ʴ�Ϊ��AC��
���� ���⿼����������ᴿʵ�鼰��Һ����ʵ�飬Ϊ��Ƶ���㣬����������ʹ�á����������ᴿ������һ��Ũ�ȵ���Һ��ʵ�����������Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע����Ũ�ȹ�ʽ��������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ؽ���������ʹ���������ᣮ������ʳ�ؽ���������ʹ���ж� | |
| B�� | �ڵ�������Һ�м��뱥���������Һ�����������������ټ�ˮ��Ҳ���ܽ� | |
| C�� | �˹��ϳɾ������������ĵ�����--�ᾧţ�ȵ������ҹ���ѧ����1965���״κϳ� | |
| D�� | Ũ���ὦ��Ƥ������ʹƤ�����ֻ�ɫ��������Ũ����͵����ʷ�������ɫ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\overline{v}$��O2��=0.3 mol•L-1•s-1 | B�� | $\overline{v}$��NO��=0.24mol•L-1•s-1 | ||
| C�� | $\overline{v}$��NH3��=0.12 mol•L-1•s-1 | D�� | $\overline{v}$��H2O��=0.36mol•L-1•min-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �Լ� | ���ӷ���ʽ | |
| ��KCl ��K2SO4�� | ||
| ��Na2SO4 ��MgSO4�� | ||
| ��NaCl ��Na2CO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����֪Ũ�ȵ�NaOH��Һ�ⶨδ֪Ũ�ȵĴ�����Һ��Ũ�ȣ�H++OH-�TH2O | |
| B�� | ʢ��NaOH��Һ���Լ�ƿ�����ò�������SiO2+2Na++2OH-�TNa2SiO3+H2O | |
| C�� | �������ȷ�Ӧ���Ӹֹ죺2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3 | |
| D�� | ����ˮ��ȥFeCl2��Һ�е�Fe2+��Cl2+Fe2+�T2Cl-+Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ԭ�ӵ����ʵ���֮��Ϊ1��1 | B�� | ��Ԫ�ص�����֮��Ϊ5��4 | ||
| C�� | ��Ԫ�ص�����֮��Ϊ5��6 | D�� | ������ԭ�ӵ����ʵ���֮��Ϊ3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��Cl-��SO42-��K+ | B�� | K+��Cu2+��SO42-��NO3- | ||
| C�� | K+��H+��HCO3-��Cl- | D�� | Mg2+��Cl-��SO42-��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com