
H2O2�ڹ�ҵ��ũҵ��ҽҩ�϶��й㷺����;��
��1���������ʶ�������H2O2�ֽ�Ĵ�����һ�ֹ۵���Ϊ���ڷ�Ӧ�����д����ȱ�H2O2��������ԭ�������ֱ�H2O2��ԭ�������������������ʶ�����H2O2�ֽ�Ĵ������ڷ�Ӧ�������ȱ���������ԭ���������������������������� ��
a�� I���� ��������b�� Fe3������ ����c�� Cu2���� ������d�� Fe2��
��2���ü�������ȼ�ϵ�غϳ�H2O2������Ч�ʸߣ�����Ⱦ���ص㡣����ܷ�ӦΪ��
H2 + O2 + OH����H2O + HO2����д��������Ӧʽ�������������������������������������� ��
��3��H2O2��һ�ֻ����Ѻõ�ǿ����������Ʒ�ˮ����Ҫ��Cu2����Ni2������������Fe3����Fe2����Cr3�� �ȣ��Ʊ���������һ���������£�

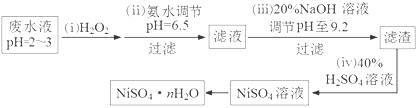
���ٵڣ�������������H2O2��Ӧ�����ӷ���ʽ������������������������������������ ��
��Ϊ�ⶨNiSO4��nH2O����ɣ���������ʵ�飺��ȡ 2.627g��Ʒ�����Ƴ�250.00 mL��Һ��ȷ��ȡ���Ƶ���Һ25.00 mL����0.04000 mol��L��1��EDTA��Na2H2Y������Һ�ζ�Ni2+�����ӷ���ʽΪNi2��+ H2Y2����NiY2��+ 2H����������EDTA����Һ25.00 mL��������������Ļ�ѧʽΪ���������� ��������������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ˮ���������H+Ũ��Ϊ1��10��13mol/L����Һ�У�һ���ܴ����������������
�� K+��Cl����NO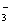 ��S2�� ��K+��Fe2+��I����SO
��S2�� ��K+��Fe2+��I����SO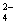 �� Na+��Cl����NO
�� Na+��Cl����NO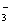 ��SO
��SO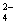
��Na+��Ca2+��Cl����HCO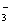 �� K+��Ba2+��Cl����NO
�� K+��Ba2+��Cl����NO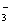
A���٢� B���ۢ� C���ۢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧС��ѧ��������ͼ����װ�ý��С�����ˮ��Ӧ����ʵ�飬�����ò����һ����ȡ
FeCl3��6H2O���塣��ͼ�мгּ�β������װ�þ�����ȥ��
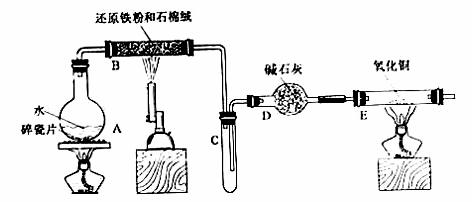
��1��װ��B�з�����Ӧ�Ļ�ѧ����ʽ��___________________________��
��2��װ��B�е�������________________________________________��
��3��ֹͣ��Ӧ����B����ȴ��ȡ���еĹ��壬�������ϡ�����ַ�Ӧ�����ˡ�����������Һ��Fe3+�IJ���������________________________��
��4����С��ѧ������������Һ��ȡFeCl3��6H2O���壬����������£�
��Һ FeCl3��Һ
FeCl3��Һ FeCl3��6H2O����
FeCl3��6H2O����
�ٲ������ͨ��Cl2��������____________________________________��
�ڲ�����FeCl3ϡ��Һ�еõ�FeCl3��6H2O�������Ҫ����������
_____________________________________________________________��
�۸��������豣�������������Ҫԭ���ǣ�������ӷ���ʽ��Ҫ˵����
_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ת�������в���ͨ��һ����Ӧʵ�ֵ���
��SiO2��Na2SiO3������ ������SiO2��H2SiO3������������CuSO4��Cu(NO3)2����
��CuO��Cu(OH)2�� ����������Cl2��NaClO������������SO2 ��H2SO4
��A���٢ڢޡ����� B���ڢۢܡ����� C���ۢܢݡ����� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������(Na2S2O3)��Һ�������ϣ����ɵ����������������������������Һ���������Լ�(�����뷴Ӧ)���Ȼ�����ձ��У��ټ���������Һ�����̽��裬���ú������������õ������ʵ�ǻ��(���ɢ����ϵ��)������������������
��A��������Լ������������ɵ����� ��B���ձ��е��������ֱ��ԼΪ 10-9-10-7m
��C����������������ᷴӦ�У�������������
��D����ƽ�й������ձ���Һ�壬���۲쵽��ġ�ͨ·��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ʵ�����Ʊ��������������ᶡ����������ȷ����
A.������ˮԡ���� B.�Ʊ���������ʱ����������
C.�����ñ߷�Ӧ������ķ��� D.�Ʊ���������ʱ�Ҵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
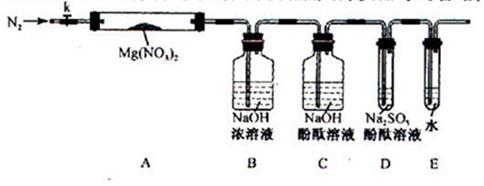
���������μ����ֽ��Ҳ���ϸ��ӡ�ijѧϰС����Mg(NO3)2Ϊ�о�������ͨ��ʵ��̽�����ȷֽ�IJ���������4�ֲ��룺
�ף�Mg(NO3)2��NO2��O2 �ң�MgO��NO2��O2 ����Mg3N2��O2 ����MgO��NO2��N2
��1��ʵ��ǰ��С���Ա�������϶����붡��������������_____________��
�������ϵ�֪��2NO2+2NaOH=NaNO3+NaNO2+H2O
��Լס��ҡ������룬�������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
��2��ʵ�����
��ȡ�����Ӻ��˹����Լ�֮ǰ���ر�k����Ӳ�ʲ����ܣ�A�����۲쵽E �������������ų�������________
�� ��ȡMg(NO3)2����3 . 79 g����A�У�����ǰͨ��N2������װ���ڵĿ�������Ŀ����________���ر�K���þƾ��Ƽ���ʱ����ȷ��������________Ȼ��̶��ڹ��й��岿λ�¼��ȡ�
�� �۲쵽A ���к���ɫ������֣�C��D ��δ�����Ա仯��
�� ����Ʒ��ȫ�ֽ⣬A װ����ȴ�����¡����������ʣ����������Ϊ1 . 0g
�� ȡ����ʣ��������Թ��У���������ˮ��δ����������
( 3 ��ʵ������������
�� ����ʵ�������ʣ�����������������ɳ���ȷ�ϲ���_______����ȷ�ġ�
�� ����D ������������һλͬѧ��Ϊ����ȷ�Ϸֽ��������O2����Ϊ����O2��D�н�����������ԭ��Ӧ��_____________________����д��ѧ����ʽ������Һ��ɫ����ȥ��С�������϶��ֽ��������O2���ڣ�δ��ൽ��ԭ����_____________________��
�� С�����ۺ��ɵĹ�ʶ������ʵ������Բ����ƣ���Ľ�װ���һ���о���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������AgNO3��BaCl2��K2SO3��Mg(NO3)2������Һ�����������Ǽ�����Ӧ�����Լ�����
A. ���ᡢ���� B. ���ᡢ����������Һ
C. ��ˮ������ D. ��ˮ������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˱�����ͭ������ͭ�̣����·�����ȷ����
A ����ͭ����������������
B ����ͭ�������ڸ���Ļ�����
C ����ͭ�������ڳ�ʪ�Ŀ�����
D ����ͭ���ı��渲��һ������ĸ߷���Ĥ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com