
����Ŀ������������![]() �����������Ͻ���Բ��ϡ������ȡ�Ϊ���������Դ����ʵ������̽�����ú��ܷ�������
�����������Ͻ���Բ��ϡ������ȡ�Ϊ���������Դ����ʵ������̽�����ú��ܷ�������![]() �ȣ����Ʊ����������ܣ������������£�
�ȣ����Ʊ����������ܣ������������£�

�ش��������⣺
��1��![]() �ĵ���ʽΪ_______________________��
�ĵ���ʽΪ_______________________��
��2��������ˮϴ����Ŀ��Ϊ______________________________________________��
��3���������ʱ��![]() ������Ӧ�����ӷ���ʽΪ_____________________________________________������1����Ҫ�ɷֵĻ�ѧʽΪ_______________________��
������Ӧ�����ӷ���ʽΪ_____________________________________________������1����Ҫ�ɷֵĻ�ѧʽΪ_______________________��
��4��������ˮϴҺ���к���![]() �IJ���������Ϊ______________________________________________�����������ж��Ӧ�ù��˲����ò���������Ҫ����������_____________________��
�IJ���������Ϊ______________________________________________�����������ж��Ӧ�ù��˲����ò���������Ҫ����������_____________________��
��5������װ���У��ʺϽ�������������������_____________________����ѡ����ĸ����
A. B.
B. C.
C. D.
D.
��6������![]() ���������������ø���������
���������������ø���������![]() ����������Ļ�ѧʽΪ________________�������������������ɵ������ܲ������ѭ������ù���_______________�������������������������μӷ�Ӧ��
����������Ļ�ѧʽΪ________________�������������������ɵ������ܲ������ѭ������ù���_______________�������������������������μӷ�Ӧ��
���𰸡�![]() ��ȥ���ܷ������������ Co2O3+4H++
��ȥ���ܷ������������ Co2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O CaSO4 �ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ �ձ���©���������� C Co2O7 ��
+2H2O CaSO4 �ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ �ձ���©���������� C Co2O7 ��
��������
��������ʾ��ͼ������֪�����ܷ������ȵĴ�����Һϴȥ��������ۣ���ˮK2O�ܽ⣬ͬˮϴҺ������ϴ�Ӻ����������Һ��Na2SO3������������ʱ������ӦCo2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O��ͬʱCaO��H2SO4��Ӧ����CaSO4���õ�����CaSO4������1����Ҫ��CoSO4����Һ1���ټ���NH4HCO3������ӦCoSO4+NH4HCO3=CoCO3+NH4HSO4�õ�CoCO3�ij��������������պ�õ�����������(CoxOy)���ݴ˷������
+2H2O��ͬʱCaO��H2SO4��Ӧ����CaSO4���õ�����CaSO4������1����Ҫ��CoSO4����Һ1���ټ���NH4HCO3������ӦCoSO4+NH4HCO3=CoCO3+NH4HSO4�õ�CoCO3�ij��������������պ�õ�����������(CoxOy)���ݴ˷������
(1)K2O�����ӻ����K+��O2-�γ����Ӽ��������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)�ȵĴ�����Һ���н�ǿ�ļ��ԣ���ʹ��������ˮ��Ϊ������ˮ�����ʣ�����ˮϴ��ȥ���ʴ�Ϊ����ȥ���ܷ�����������ۣ�
(3)�������ʱ��Co2O3�����������±���ԭ����Ӧ�����ӷ���ʽΪCo2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O��ͬʱ��CaO��H2SO4��Ӧ�����ܽ��С��CaSO4���ʴ�Ϊ��Co2O3+4H++
+2H2O��ͬʱ��CaO��H2SO4��Ӧ�����ܽ��С��CaSO4���ʴ�Ϊ��Co2O3+4H++![]() =2Co2++
=2Co2++![]() +2H2O��CaSO4��
+2H2O��CaSO4��
(4)����K+������ɫ��Ӧ������������Ϊ�ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ���������õ���Ҫ�����������ձ���©���Ͳ��������ʴ�Ϊ���ýྻ��˿պȡ����Һ�ھƾ��ƻ��������գ�����ɫ�ܲ����۲�������ɫ���ձ���©������������
(5)�����ա�����Ӧ�������н��У�Cѡ����ȷ���ʴ�Ϊ��C��
(6)5.95gCoCO3�����ʵ���Ϊ![]() �����������к���Ԫ�ص�����Ϊ0.05mol��59g��mol-1=2.95g������Ԫ�ص�����Ϊ4.07g-2.95g=1.12g����0.07mol����˸�����������Co��O�ĸ�����Ϊ5��7���������ܵĻ�ѧʽΪCo2O7��CoCO3ת��ΪCo5O7ʱCoԪ�صĻ��ϼ����ߣ����������μӷ�Ӧ���ʴ�Ϊ��Co2O7���С�
�����������к���Ԫ�ص�����Ϊ0.05mol��59g��mol-1=2.95g������Ԫ�ص�����Ϊ4.07g-2.95g=1.12g����0.07mol����˸�����������Co��O�ĸ�����Ϊ5��7���������ܵĻ�ѧʽΪCo2O7��CoCO3ת��ΪCo5O7ʱCoԪ�صĻ��ϼ����ߣ����������μӷ�Ӧ���ʴ�Ϊ��Co2O7���С�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B�ֱ�Ϊ��3��4����ͬһ����IJ�ͬԪ�ص�ԭ�ӣ�����ԭ�Ӻ��ڵ�����������������������AΪ�ڢ�A��Ԫ�أ���������Ϊx����B��������Ϊy������AΪ�ڢ�A��Ԫ�أ���������Ϊm����B��������Ϊn����y��n��ֵ�ֱ��ǣ�������
A.��![]() ��18������2m��18��
��18������2m��18��
B.��![]() ��8������2m��18��
��8������2m��18��
C.��![]() ��8������2m��36��
��8������2m��36��
D.��![]() ��18������2m��36��
��18������2m��36��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ�����о�DZˮͧ�й�����ϵ��Ӧ������װ��ͼ(�г�������)��
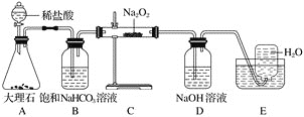
��1��Aװ��ΪCO2�ķ���װ�ã���Ӧ�����ӷ���ʽΪ____________________��
��2��Bװ�ÿɳ�ȥAװ���п��ܻӷ�����___________����Ӧ�����ӷ���ʽΪ_______________��
��3��Cװ��ΪO2�ķ���װ�ã���Ӧ�Ļ�ѧ����ʽΪ__________________��________________��
��4��Dװ�ÿɳ�ȥCװ����δ��Ӧ��__________����Ӧ�����ӷ���ʽΪ____________________��
��5��Eװ��Ϊ��ˮ���ռ�O2��װ�ã��������ռ�������ΪO2�ķ���Ϊ_____________________��
��6��Cװ���й����ɵ���ɫ��ȫ��Ϊ��ɫ���������ɷֵ�ʵ�鷽��Ϊȡ����Cװ���з�Ӧ��Ĺ�������ˮ������Һ�е������___��Һ�����а�ɫ�������ɣ���֤�������к���___�����ˣ�����Һ�е��뼸�η�̪��Һ����__�Ҳ���ɫ����֤�������к���__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ����101 k Paʱ��ǿ����ǿ���ϡ��Һ�����кͷ�Ӧ���к���Ϊ57.3 kJ/mol�������ȼ����Ϊ5518 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ���ǣ�������
A. 2H+(aq) +![]() (aq)+
(aq)+![]() (aq)+2OH
(aq)+2OH![]() (aq)=BaSO4(s)+2H
(aq)=BaSO4(s)+2H![]() O(l);
O(l);![]() H=
H=![]() 57.3 kJ/mol
57.3 kJ/mol
B. KOH(aq)+![]() H
H![]() SO4(aq)=
SO4(aq)=![]() K
K![]() SO4(aq)+H
SO4(aq)+H![]() O(l);
O(l);![]() H=
H=![]() 57.3kJ/mol
57.3kJ/mol
C. C8H18(l)+![]() O
O![]() (g)=8CO
(g)=8CO![]() (g)+ 9H
(g)+ 9H![]() O;
O;![]() H=
H=![]() 5518 kJ/mol
5518 kJ/mol
D. 2C8H18(g)+25O![]() (g)=16CO
(g)=16CO![]() (g)+18H
(g)+18H![]() O(l);
O(l);![]() H=
H=![]() 5518 kJ/mol
5518 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�л���������ͼת����ϵ��C�ں˴Ź��������г���4��壬������֮��Ϊ6:2:1:1���ش��������⣺
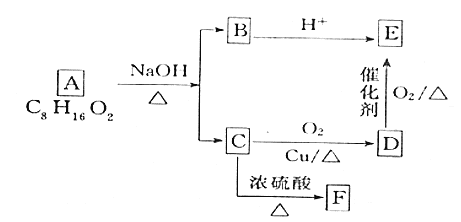
(1)C���ʵ�ͬϵ���У���һ�����ʵ�Ũ��Ϊ![]() ����Һ���ܴﵽ���õ�������ɱ�����ã������ʵ�������________��
����Һ���ܴﵽ���õ�������ɱ�����ã������ʵ�������________��
(2)D�Ĺ����ŵ�������________��
(3)�����л������ܸ�![]() ��Ӧ����_______________(����ĸ)��
��Ӧ����_______________(����ĸ)��
(4)��A����B��C�Ļ�ѧ��Ӧ����ʽΪ____��
(5)��֪F��ʹ������Ȼ�̼��Һ��ɫ��G��Fͬϵ����������ʣ������G�ͶԱ�������Ϊԭ�Ͼ�3����Ӧ�Ʊ��Ա��������Ҷ������ĺϳ�·��(�л���д�ṹ��ʽ�������Լ���ѡ)_________
��.����J�Ǿ���ǿ��õ�����������ϣ��������з�Ӧ·�ߺϳ�(���ַ�Ӧ��������)
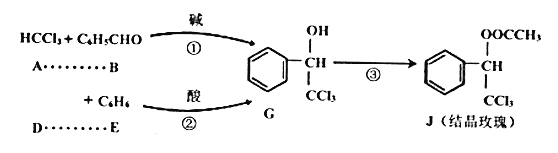
(1)A���ʵ������_______����Ӧ���Ǽӳɷ�Ӧ����D���ʵĽṹ��ʽ��_______��
(2)�۵ķ�Ӧ����Ϊ_______��
(3)G��ͬ���칹��L��![]() ��Һ����ɫ��������������ˮ��Ӧδ����ɫ������������L�Ľṹ��ʽΪ_______��(ֻдһ��)
��Һ����ɫ��������������ˮ��Ӧδ����ɫ������������L�Ľṹ��ʽΪ_______��(ֻдһ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����¶�Ϊ20 �棬Ũ��Ϊ1.0 mol/L��H2SO4��Һ��2.2 mol/L�ļ���Һ��50 mL���[��Һ�ܶȾ�Ϊ1g/mL��������Ϊ4.18 kJ/(kg����)]�����������������Һ���¶ȱ仯�������£�
��Ӧ�� | ��ʼ�¶�t1 �� | ��ֹ�¶�t2 �� |
H2SO4��NaOH | 20 | 33.6 |
H2SO4��NH3��H2O | 20 | 32.6 |
��1����ӦNH3��H2O(aq)![]() NH4+ (aq)��OH��(aq)���ʱ�Լ____��
NH4+ (aq)��OH��(aq)���ʱ�Լ____��
��2��������������ʵ�������к��ȣ���H1��___kJ/mol����H2��__kJ/mol��
��3���ɱ�����ۿ�Ԥ�⽫��1���е�1 mol/L��H2SO4��Һ����2mol/L��CH3COOH��Һ����ʵ�飬��õ��к�����ֵ__(������������С��������������)56.848��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪þ����O2��N2��CO2��ȼ��������Ӧ�Ļ�����Իش�
(1)þ�ڿ�����ȼ�գ���������ӦN2��3Mg![]() Mg3N2�⣬���ܷ���������Ӧ��д����Ӧ�Ļ�ѧ����ʽ��____________��
Mg3N2�⣬���ܷ���������Ӧ��д����Ӧ�Ļ�ѧ����ʽ��____________��
(2)þ����������ȼ��ʱ������������________��
(3)�����������þ�ֱ���������������������������̼��ȼ�գ�ȼ�պ����������ֱ�Ϊm1��m2��m3�������ǵĴ�С˳��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ������С��������ͼ��ʾװ�÷ֱ�������ʵ�飺
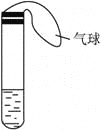
��1�����Թ���ע��ij��ɫ��Һ�������Թܣ���Һ��ɫ��dz����ȴ��ָ���ɫ����ԭ��Һ������________��Һ������ʱ��Һ�ɺ�ɫ��dz��ԭ����________________��
��2�����Թ���ע��ij��ɫ��Һ�������Թܣ���Һ��Ϊ��ɫ����ȴ��ָ���ɫ�������Һ������________��Һ������ʱ��Һ����ɫ��Ϊ��ɫ��ԭ����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1molI2��2molH2(g)����ij2L�ܱ������У��������¶��·�����Ӧ��I2(g)+H2(g)![]() 2HI(g) ��H<0������ƽ�⣬HI�����������(HI)��ʱ��仯��ͼ����
2HI(g) ��H<0������ƽ�⣬HI�����������(HI)��ʱ��仯��ͼ����

(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ _______��H2(g)��ƽ��ת����Ϊ___________��
�ڴ��¶��£��÷�Ӧ��ƽ�ⳣ��K__________(����һλС������
(2)���ı䷴Ӧ�����£��ڼ������¦�(HI)�ı仯��ͼ����(I)��ʾ�����������¦�(HI)�ı仯��ͼ����(III)��ʾ���������������______������������������š���ͬ����������������________��
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��º��������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���a mol I2(g)��b mol H2��c mol HI��a��b�� c������0����������Ӧ����ƽ��ʱ��HI�����������Ϊ0.60����a��b��c��Ӧ����Ĺ�ϵ��_______________ (�ú�һ��a��b��c�Ĵ���ʽ��ʾ)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com