
����Ŀ��SO2��Σ����Ϊ���صĴ�����Ⱦ��֮һ��SO2�ĺ����Ǻ���������Ⱦ��һ����Ҫָ�ꡣ��ҵ�ϳ����ô���ԭ�������շ�����SO2������ԭSO2������������SO2��Ⱦ�����ҿɵõ��м�ֵ�ĵ���S��
��1����֪CH4��S��ȼ���ȷֱ�Ϊa kJ/mol ��b kJ/mol���ڸ�����ִ��������£�CH4��ʹSO2ת��ΪS��ͬʱ����CO2 ��Һ̬ˮ����Ӧ���Ȼ�ѧ����ʽΪ______________������H�ú�a��b�Ĵ���ʽ��ʾ��
��2����H2��ԭSO2����S�ķ�Ӧ��������ɣ���ͼ1��ʾ���ù�����������ʵ����ʵ���Ũ����ʱ��ı仯��ϵ��ͼ2 ��ʾ��

�ٷ�����֪XΪ_______________��д��ѧʽ����0~t1ʱ��εķ�Ӧ�¶�Ϊ_______________��0~t1ʱ�����SO2��ʾ�Ļ�ѧ��Ӧ����Ϊ______________________��
���ܷ�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��3����̿����ԭSO2����S2�Ļ�ѧ����ʽΪ��2C(s)+2SO2(g)![]() S2(g)+2CO2(g)�����������У�lmol/L SO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3 ��ʾ��
S2(g)+2CO2(g)�����������У�lmol/L SO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3 ��ʾ��
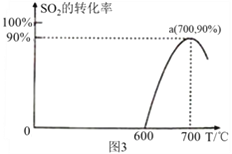
�ٸ÷�Ӧ�Ħ�H______0���������������
��700���ƽ�ⳣ��Ϊ_____________________��
��4����ҵ�Ͽ���Na2SO3��Һ����SO2���÷�Ӧ�����ӷ���ʽΪ___________��25��ʱ��1mol/L��Na2SO3��Һ����SO2������ҺpH=7ʱ����Һ�и�����Ũ�ȵĴ�С��ϵΪ___________������֪��H2SO3�ĵ��볣��K1=1.3��10-2��K2=6.2��10-8��
���𰸡� CH4 (g)+2SO2 (g) = CO2 (g)+2S (s)+2H2O(l) ��H=��2b-a�� kJ/mol H2S 300�� 2��10-3/t1 mol/(L�� min) 2H2+SO2 = S+2H2O �� 36.45 mol/L SO32- +SO2 + H2O = 2HSO3- c(Na+)��c(HSO3-)��c(SO32-)��c(H+)=c(OH-)
�����������������⿼���˹���ɵ�Ӧ�á��Ȼ�ѧ����ʽ����д����ѧ��Ӧ���ʵļ��㡢����ʽ����д����ѧƽ��ͼ��ķ����ͼ��㡢��Һ������Ũ�ȵĴ�С��ϵ��
��1������ȼ���ȵĸ���д���Ȼ�ѧ����ʽ�����ø�˹���ɡ�
��2������ͼʾ��ͼ�������ʵı仯�Լ����ʵ���Ũ��֮��Ĺ�ϵ�����ԭ���غ㣬XΪH2S��0~t1ʱ��εķ�Ӧ�¶�Ϊ300�档����ͼ��ͻ�ѧ��Ӧ���ʵı���ʽ�����ԣ�SO2��������ͼʾд���ܷ�Ӧ��
��3������ͼ��a��ﵽƽ��״̬�������¶�SO2��ƽ��ת���ʼ�С��ƽ�����淴Ӧ�����ƶ�������H![]() 0������ͼ��ת���ʺ�����ʽ���㻯ѧƽ�ⳣ����
0������ͼ��ת���ʺ�����ʽ���㻯ѧƽ�ⳣ����
��4��Na2SO3����SO2�����ӷ���ʽΪSO32-+SO2+H2O=2HSO3-��Na2SO3��Һ�ʼ��ԣ�NaHSO3��Һ�����ԣ���Na2SO3��Һ����SO2�õ�pH=7����Һ����Һ������ΪNa2SO3��NaHSO3����Һ��c��H+��=c��OH-��=1![]() 10-7mol/L������K2��H2SO3��=
10-7mol/L������K2��H2SO3��=![]() =6.2
=6.2![]() 10-8����
10-8����![]() =6.2
=6.2![]() 10-8
10-8![]() ��1
��1![]() 10-7��=0.62��c��SO32-��
10-7��=0.62��c��SO32-��![]() c��HSO3-������Һ������Ũ���ɴ�С��˳��Ϊc��Na+��
c��HSO3-������Һ������Ũ���ɴ�С��˳��Ϊc��Na+��![]() c��HSO3-��
c��HSO3-��![]() c��SO32-��
c��SO32-��![]() c��H+��=c��OH-����
c��H+��=c��OH-����
�������1��CH4��S��ȼ���ȷֱ�ΪakJ/mol��bkJ/mol��д���Ȼ�ѧ����ʽ��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-akJ/mol���٣�
S��s��+O2��g��=SO2��g����H=-bkJ/mol���ڣ�
Ӧ�ø�˹���ɣ�����-��![]() 2����CH4��g��+2SO2��g��=CO2��g��+2S��s��+2H2O��l����H=��2b-a��kJ/mol��
2����CH4��g��+2SO2��g��=CO2��g��+2S��s��+2H2O��l����H=��2b-a��kJ/mol��
��2��������ͼʾ��ͼ��һ����Ӧͼ��0~t1������H2��SO2��Ӧ����X����Ӧ���ĵ�H2��SO2�����ɵ�X���ʵ���Ũ��֮��Ϊ��6![]() 10-3������3
10-3������3![]() 10-3-1
10-3-1![]() 10-3������2
10-3������2![]() 10-3��=3:1:1���ڶ�����Ӧͼ��t1~t2����X��SO2��Ӧ����S�����ĵ�X��SO2���ʵ���Ũ��֮��Ϊ��2
10-3��=3:1:1���ڶ�����Ӧͼ��t1~t2����X��SO2��Ӧ����S�����ĵ�X��SO2���ʵ���Ũ��֮��Ϊ��2![]() 10-3������1
10-3������1![]() 10-3��=2:1�����ԭ���غ㣬XΪH2S��0~t1ʱ��εķ�Ӧ�¶�Ϊ300�档�ԣ�SO2��=
10-3��=2:1�����ԭ���غ㣬XΪH2S��0~t1ʱ��εķ�Ӧ�¶�Ϊ300�档�ԣ�SO2��=![]() =
=![]() =
=![]() mol/��L��min����
mol/��L��min����
������ͼʾ��ͼ���ܷ�ӦΪH2��SO2�����ʵ���֮��2:1��Ӧ����S�����ԭ���غ㣬�ܷ�Ӧ�Ļ�ѧ����ʽΪ2H2+SO2=S+2H2O��
��3��������ͼ��a��ﵽƽ��״̬��700���������¶�SO2��ƽ��ת���ʼ�С��ƽ�����淴Ӧ�����ƶ����淴ӦΪ���ȷ�Ӧ����÷�Ӧ����H![]() 0��
0��
��700��ʱSO2��ת����Ϊ90%��������ʽ
2C��s��+2SO2��g��![]() S2��g��+2CO2��g��
S2��g��+2CO2��g��
c����ʼ����mol/L�� 1 0 0
c��ת������mol/L�� 1![]() 90% 0.45 0.9
90% 0.45 0.9
c��ƽ�⣩��mol/L�� 0.1 0.45 0.9
700���ƽ�ⳣ��K=![]() =
=![]() =36.45mol/L��
=36.45mol/L��
��4��Na2SO3����SO2�����ӷ���ʽΪSO32-+SO2+H2O=2HSO3-��Na2SO3��Һ�ʼ��ԣ�NaHSO3��Һ�����ԣ���Na2SO3��Һ����SO2�õ�pH=7����Һ����Һ������ΪNa2SO3��NaHSO3����Һ��c��H+��=c��OH-��=1![]() 10-7mol/L������K2��H2SO3��=
10-7mol/L������K2��H2SO3��=![]() =6.2
=6.2![]() 10-8����
10-8����![]() =6.2
=6.2![]() 10-8
10-8![]() ��1
��1![]() 10-7��=0.62��c��SO32-��
10-7��=0.62��c��SO32-��![]() c��HSO3-������Һ������Ũ���ɴ�С��˳��Ϊc��Na+��
c��HSO3-������Һ������Ũ���ɴ�С��˳��Ϊc��Na+��![]() c��HSO3-��
c��HSO3-��![]() c��SO32-��
c��SO32-��![]() c��H+��=c��OH-����
c��H+��=c��OH-����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п��һ����Ҫ�Ĺ��ɽ�����п���仯�������Ź㷺��Ӧ�á�
(1)ָ��п�����ڱ��е�λ�ã���________���ڣ���________�壬________����
(2)Zn�γɽ������壬�����ԭ�Ӷѻ���������______ģʽ��
A�������� B������ C��þ�� D��ͭ��
(3)��������п[CH2OH(CHOH)4COO]2Zn��Ŀǰ�г������еIJ�п����д��Zn2����̬�����Ų�ʽ________�������Ƿ�����̼ԭ�ӵ��ӻ���ʽΪ________��
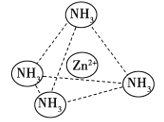
(4)Zn2������NH3�γ�������[Zn(NH3)4]2������λ��NH3��������________(����Է��ӡ��Ǽ��Է��ӡ�)����[Zn(NH3)4]2���У�Zn2��λ�������������ģ�NH3λ����������Ķ��㣬��������ͼ�б�ʾ[Zn(NH3)4]2����Zn2����NH3֮��Ļ�ѧ��______��
(5)��ͼ��ʾп��ij�ǽ���Ԫ��X�γɵĻ����ᄃ��������Zn��Xͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ______________________���û����ᄃ����۵�ȸɱ��ߵö࣬ԭ����____________________��

(6)��пͬ���ڣ��������3��δ�ɶԵ��ӵ�Ԫ��������________����Ԫ������������Ӧˮ��������Ա��������������Ӧˮ���������________(�ǿ��������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij�ݻ��̶����ܱ������ɿ��ƶ��Ļ�������A��B���ң��ֱ���A��B���ҳ���H2��O2�Ļ�������1 mol��������ʱ������λ����ͼ��ʾ����ʵ����A�һ�����������Ϊ34 g��������˵���в���ȷ���ǣ� ��
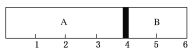
A. A���л������������������Ϊ2NA
B. A���л��������ܶ���ͬ��ͬѹ�������ܶȵ�8.5��
C. A���л��������H2��O2�����ʵ���֮��Ϊ1:1
D. ��A��H2��O2�Ļ�������ȼ�������ָ������º����ջ���ǡ��ͣ����3�̶ȴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ�����������������
A. �����£�pH=12��Ba(OH)2��Һ��Ba2+����ĿΪ5��10-3NA
B. 14g��ϩ��14g��ϩ�к��еĹ��õ��Ӷ�������3NA
C. 1 mol FeI2��һ����������Ӧʱ������0.5mol Fe2+��������ת�Ƶĵ�����Ϊ2.5NA
D. 0.4mol AgNO3������ȫ�ֽ⣨2AgNO3![]() 2Ag+2NO2��+O2����������ˮ�������ռ�������ķ�����Ϊ0.1NA
2Ag+2NO2��+O2����������ˮ�������ռ�������ķ�����Ϊ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ��2NO2(g) ![]() 2NO(g)��O2(g)��һ���ݵ��ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��( )
2NO(g)��O2(g)��һ���ݵ��ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��( )
�ٵ�λʱ��������n mol O2��ͬʱ����2n mol NO2 �ڵ�λʱ��������n mol O2��ͬʱ����2n mol NO ����NO2��NO��O2�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ2��2��1��״̬ �ܻ���������ɫ���ٸı��״̬ �ݻ��������ܶȲ��ٸı��״̬ ��������ƽ����Է����������ٸı��״̬ ��������ѹǿ���ٸı��״̬
A. �٢ܢޢ� B. �ڢۢݢ� C. �٢ۢܢ� D. �٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ÿ������һϵ��ʵ�飨ͼ�мг�װ������ȥ��

��ش��������⣺
��. ������a��Cl2��װ��A��B��C�е��Լ�����Ϊ�� FeCl2��Һ������KI��Һ��ʯ����Һ��
��1��A��Һ��dz��ɫ��Ϊ�ػ�ɫ����Ӧ�����ӷ���ʽΪ_________________________________��
��2��Bƿ�еĵ���KI��Һ��Ϊ��ɫ����Ӧ�����ӷ���ʽΪ______________________________��
��3��Cƿ�е�����Ϊ_______________________________________________________________��
��4��Dװ�����Լ�Ϊ_______________________________________________________________��
II��������a��SO2��װ��A��B��C�е��Լ�����Ϊ��Ʒ����Һ�����Ը��������Һ�������ᣨ�����ˮ��Һ����
��1��Aƿ��Ʒ���Bƿ�����Ը��������Һ����ɫ������������SO2Ư���Ե��� _____����д���A��B����
��2��Cƿ�в�������ɫ������ ��Ӧ�Ļ�ѧ����ʽ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪһ�ֹ������ӵ�������ʪ��KI��ֽAB���ӣ�Ag+������RbAg4I5������Ǩ�ƣ������е���������������ϩĤ��AlI3��Ӧ����I2��Ag��I2�����γɵ�ء�����˵������ȷ���ǣ� ��
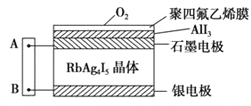
A. ��ֽB�˷���������Ӧ B. Ag+��ʯī�缫�������缫
C. ��ֽA�˷�����Ӧ��2I--2e-=I2 D. ���õ��ת��1mol���ӣ�����ֽ������8gO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������1.000 mol��L��1�������20.00 mL 1.000 mol��L��1��ˮ������ҺpH���¶��������������仯������ͼ��ʾ�������й�˵����ȷ����(����)

A. a����ˮ�������c(H��)��1.0��10��14 mol��L��1
B. b����c(NH![]() )��c(NH3��H2O)��c(Cl��)
)��c(NH3��H2O)��c(Cl��)
C. c����c(Cl��)��c(NH![]() )
)
D. d�������Һ�¶����½�����Ҫԭ����NH3��H2O��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ϊ�ⶨ��������Ʒ�������� Na2O�����Ƶ��ʵ��������������������ʵ�飨��Ӧװ����ͼ��ʾ����

�ٳ��� A��B ��������
�ڳ�ȡһ������������Ʒ
�۽�����ƷͶ����ƿ�У�Ѹ�������� U �θ���ܣ��ں���ˮ CaCl2 ���� ��������Ƥ�� ���й������ǣ���ȡ�Ľ�������Ʒ����Ϊ a g��A��B ��Ӧǰ������Ϊ b g����Ӧ�� A��B ��������Ϊ c g�� ���������ش��������⣺
��1��A ���ƺ�ˮ��Ӧ���̿ɿ������۳�������С������һ����ԭ���ǣ�_____________
��2���� a��b��c ��ʾ���Ƶ��ʵ���������Ϊ_______________
��3�����û�� B װ�ö�ʵ�����к�Ӱ��___________�����ƫ��ƫС������Ӱ�족��
�����ý����ƺͿ����Ʊ����Ƚϸߵ� Na2O2�������õ�װ������ͼ���ش��������⣺


��4������װ�â���ʢ�ŵ��Լ���______��Ϊ���ʵ��Ӧ��װ�â�����_____����д��ĸ�ţ���
A��I ֮ǰ B�� I �� II ֮�� C�� II �� III ֮�� D��III ֮��
��5����ȼ�ƾ��ƺ۲쵽װ�� II �е�����Ϊ_____��
�������Ƶô��Ƚϸߵ� Na2O2�������ʵ�顣�ش��������⣺
��6������֬�ް�סNa2O2��ĩ������ʯ�����ϣ�ͨ��ϸ������֬���д�CO2����֬��_______(��ܡ����ܡ�)ȼ��������
��7��ʢ��0.78 g Na2O2��1.68g NaHCO3�Ĺ�����������ܱ������м���,����������Ϊ_________g.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com