
����Ŀ��Ԫ��A��B��C��D��E��ԭ���������������Ҿ�С��36��A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�D�ļ۵�����Ϊ2����Eͬ���ڣ�E�Ļ�̬ԭ�ӵ��ڲ����ܲ����������4s�ܼ���1�������ӡ��ش��������⣺
(1)��̬Eԭ�ӵļ۵����Ų�ʽΪ____________________
(2)A��B��C����Ԫ�ص�һ�������ɴ�С��˳��Ϊ___________����Ԫ�ط��ű�ʾ����
(3)��A�ĵ��ʷ��ӻ�Ϊ�ȵ�����ķ��Ӻ����ӷֱ���________���÷��Ӻ����ӷ��ű�ʾ����AB2�Ŀռ乹��Ϊ___________������Aԭ�ӵ��ӻ�������_______________
(4)BԪ�ؼ��⻯��ķе���ͬ��Ԫ������ߵģ�ԭ����_____________
(5)��EԪ�ص���������Һ�м��������ˮ���ɵõ�����ɫ����Һ������Һ�м����Ҵ�������������ɫ���塣�þ���Ļ�ѧʽΪ[Cu(NH3)4]SO4H2O�����������ӵĽṹʽΪ____________
(6)C��D�γɻ�����ľ����ṹ��ͼ��ʾ����֪������ܶ�Ϊ��g/cm�������ӵ�����ΪNA�����߳�a=______________cm(�ú�����NA�ļ���ʽ��ʾ����

���𰸡�3d104s1 F��N��O CO��CN-(��C22-) ƽ�������� sp2 B���⻯��ΪH2O��ˮ���Ӽ������� 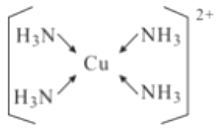
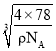
��������
Ԫ��A��B��C��D��E��ԭ���������������Ҿ�С��36��A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p3����A��NԪ�أ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ���C��ԭ����������A����ԭ�Ӻ�������Ų�Ϊ1s22s22p5������C��FԪ�أ����ԭ����������֪B��OԪ�أ�E�Ļ�̬ԭ�ӵ��ڲ����ܲ����������4s�ܼ���1�������ӣ���ԭ������С��36����E���ڵ������ڣ����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1����E��CuԪ�أ�D��Eͬ���ڣ��۵�����Ϊ2����D��CaԪ�أ��ݴ˽��
(1)���ݷ�����֪E��ͭԪ�أ����ݹ���ԭ��֪�����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1���ʻ�̬Cuԭ�ӵļ۵����Ų�ʽΪ��3d104s1��
(2)ͬһ�����У�Ԫ�صĵ�һ����������ԭ�������������������VA��Ԫ�ص�һ�����ܴ��ڵ�VIA��Ԫ�أ�����A��B��C��Ԫ�ص�һ�������ɴ�С��˳��ΪF��N��O��
(3)A��NԪ�أ�A�ĵ��ʷ���Ϊ����(N2)��2��ԭ�ӹ��ɣ��۵�����Ϊ10����Ϊ�ȵ�����ķ��Ӻ����ӷֱ���CO��CN-��C22-��AB2���ɵ�����ΪNO2��Nԭ�ӵijɼ����Ӷ���Ϊ2���µ��Ӷ���=![]() ��(5-2��2)=0.5����������ŵ��ӶԲ�����������ʱӦ����1���Դ�����Ϊ��������ҲҪռ��һ���¶Ե��ӹ����NO2�ļ۲���Ӷ�=2+1������NO2��VSEPRģ��Ϊƽ�������Σ�Nԭ�ӵ��ӻ�������sp2�ӻ���
��(5-2��2)=0.5����������ŵ��ӶԲ�����������ʱӦ����1���Դ�����Ϊ��������ҲҪռ��һ���¶Ե��ӹ����NO2�ļ۲���Ӷ�=2+1������NO2��VSEPRģ��Ϊƽ�������Σ�Nԭ�ӵ��ӻ�������sp2�ӻ���
(4)B��OԪ�أ�OԪ�ص��⻯��ķе���ͬ��Ԫ������ߵģ�����Ϊˮ���Ӽ����γ����������е���ߣ�
(5)����Ļ�ѧʽΪ[Cu(NH3)4]SO4H2O��������ͭ������NH3֮��Ļ�ѧ��Ϊ��λ�����ṹʽΪ ��
��
(6)C��FԪ�أ�D��CaԪ�أ�F��Ca�γɵĻ�����ΪCaF2���ɾ����ṹ��֪�������а�ɫ����Ŀ=8����ɫ����Ŀ=8��![]() +6��
+6��![]() =4����ɫС��ΪF����ɫ����ΪCa��������=
=4����ɫС��ΪF����ɫ����ΪCa��������=![]() �����������Ϊ��a3=
�����������Ϊ��a3=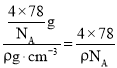 cm3�����Ըþ����߳�a=
cm3�����Ըþ����߳�a=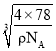 cm��
cm��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л����������ȷ����
A.CH2��CH��CH��CH2 1,3-����ϩ
B.CH3CH2CH(CH3)OH 2-��-1-����
C.![]() 2-��-3-��Ȳ
2-��-3-��Ȳ
D.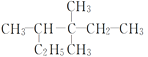 3,3,4-��������
3,3,4-��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2020��2��17�����磬�ڹ���Ժ�������ػ��Ʒ������ϣ��Ƽ����������ĸ����������ٸ����ߣ�������୶���COVID-19������������ȷ����Ч����ҩ�����ж������ҩ�����ڹ㷺��Ⱥ���Ƶİ�ȫ���ǿɿصġ���ϳ�·����ͼ��ʾ��

��֪��ȩ����һ�������¿��Ի�ԭ�ɼ����ش��������⣺
(1)�л���AΪ��ȩ�����㷺�����ڸ���ũ����Ʒ�С�A�к��������ŵ�����Ϊ______��A������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ_______��
(2)C�Ľṹ��ʽΪ________��D��E֮��Ĺ�ϵΪ_______��
(3)��Ӧ�ߵķ�Ӧ����Ϊ_______����Ӧ�����¶ȹ��ᷢ������Ӧ�����л�������Ľṹ��ʽΪ____��
(4)�л���E�ж���ͬ���칹�壬�������������������______�֣�����Щͬ���칹���У���һ�������ᣬ�Һ�������̼ԭ�ӣ�������Ϊ_________��
(5)��2�������ͱ�Ҫ���Լ��ϳ�2������[CH3CH(NH2)CH3]��_________(�ü�ͷ��ʾת����ϵ����ͷ��ע���Լ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС�鰴����ʵ������̽�������е⺬���IJⶨ�͵����ȡ��
ʵ��һ �⺬���IJⶨ
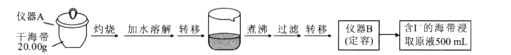
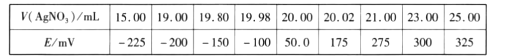
ȡ0.0100 mol/L�� AgNO3����Һװ��ζ��ܣ�ȡ100.00 mL ������ȡԭҺ���ζ��أ��õ��Ƶζ����ⶨ�⺬������õĵ綯�ƣ�E)��ӳ��Һ��c(I-)�ı仯�������������±���
�ش��������⣺
(1)ʵ����������������___________�����������ƣ�����ɵġ�
(2)�����־������������������̣�__________________________________
(3)���ݱ��������жϵζ��յ�ʱ��ȥAgNO3��Һ�����Ϊ___________mL,����ú����е�������ٷֺ���Ϊ_______________________����
ʵ��� �����ȡ
���ƺ�����ȡԭҺ���ס�������ʵ�鷽�����£�
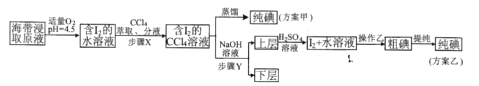
��֪��3I2+6NaOH=5NaI+NaIO3+3H2O
(4)������O2�����ܴ���O2��������ʶ�Ӧ�ĵ���ʽΪ_________________
(5)��Ҫ�ⶨ��I2+ˮ��Һ���е�ĺ���������ѡ��______________��ָʾ������ Na2S2O3��Һ�ζ����ζ��յ��������____________________��
(6)���õ���I2+ˮ��Һ��ʱ����������Һ���࣬��Na2S2O3��Һ�ζ�ʱ��������Ե�������������ԭ��Ϊ_________________�������ӷ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾһЩ�����е�ijЩ�ṹ����ش��������⣺
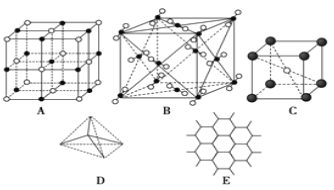
��1���������ʯ����(������ĸ����ͬ)_____������ÿ��̼ԭ����_____��̼ԭ������Ҿ�����ȡ�
��2������ʯī����_____��ÿ����������ռ�е�̼ԭ����ƽ��Ϊ_____����
��3������ NaCl ����_________��ÿ��Na+��Χ��������Ҿ�����ȵ�Cl-��_____����
��4������ CsCl ����_____��ÿ��Cs+��_____��Cl-���ڡ�
��5�������ɱ�����_____��ÿ�� CO2 ������_____�� CO2 ���ӽ��ڡ�
��6����֪ʯī��̼̼���ļ����Ƚ��ʯ��̼̼���ļ����̣����������������۵��ɸߵ��͵�����˳��Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
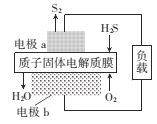
����Ŀ��H2S��һ�־綾���壬��ͼΪ����ĤH2Sȼ�ϵ�ص�ʾ��ͼ���ɶ�H2S������Դ�����á����������������
![]()
A. a�Ǹ�������ع���ʱ�����ӵ����������ǣ��缫a-����һ�缫b-����Ĥһ�缫a
B. ��ع���ʱ����ѧ��ת��Ϊ���ܺ�����
C. �缫b�Ϸ����ĵ缫��ӦʽΪO2+ 4e-+4H+=2H2O
D. ����·��ͨ��4mol����ʱ����4molH+������Ĥ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ϊ����˵����ȷ����(����)
A. Һ̬�������д����������������ӱ��Ȼ�����ȶ�
B. ��������ڷ���֮�䣬Ҳ�����ڷ���֮��
C. ���ڷ��ӣ��䷶�»���ֻ������Է������������������
D. NH3��������ˮ��CH4������ˮ��ԭ��ֻ��NH3�Ǽ��Է��ӣ�CH4�ǷǼ��Է���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
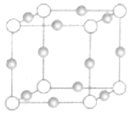
����Ŀ��Ti��Fe��Cu��NiΪ���ɽ���Ԫ�أ��ڹ�ҵ����������Ҫ��Ӧ�ã�
��1���ٳ�����Fe(CO)5��Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�森�ݴ˿����ж��侧��Ϊ_________���壬Fe(CO)5�����Ļ��ϼ�Ϊ0����������к��еĻ�ѧ��������______������ĸ����
A ���Ӽ� B ���Թ��ۼ� C �Ǽ��Թ��ۼ� D ��λ��
��NO�ܱ�FeSO4��Һ�������������[Fe(NO)(H2O)5]SO4����������������ӵ���λ��Ϊ________������H2O��Oԭ�ӵ��ӻ���ʽΪ________��
��NiO��FeO�ľ���ṹ���;��������Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO_______FeO������<������>������
��2��Cu��N��Ԫ���γ�ij�ֻ�����ľ����ṹ��ͼ����ɫ���ʾCuԭ�ӣ�����֪���ڵİ��������֮��ľ���Ϊacm���þ������ܶ�Ϊ________gcm-3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com